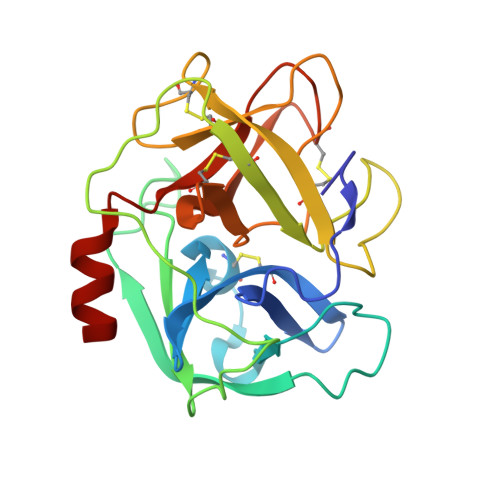
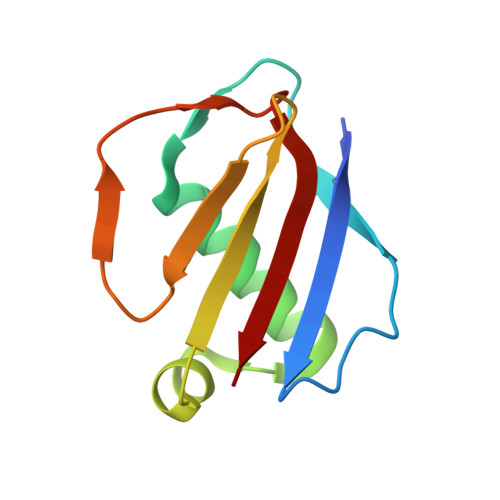
Characterization of two distinct neutrophil serine protease-binding modes within a Staphylococcus aureus innate immune evasion protein family.
Gido, C.D., Herdendorf, T.J., Geisbrecht, B.V.(2023) J Biological Chem 299: 102969-102969
- PubMed: 36736422
- DOI: https://doi.org/10.1016/j.jbc.2023.102969
- Primary Citation of Related Structures:
8D4O, 8D4Q, 8D4S, 8D4U, 8D4V - PubMed Abstract:
Extracellular adherence protein domain (EAPs) proteins are a class of innate immune evasion proteins secreted by the human pathogen Staphylococcus aureus. EAPs are potent and selective inhibitors of cathepsin-G (CG) and neutrophil elastase (NE), which are the two most abundant neutrophil serine proteases (NSPs). Previous work from our group has shown that the prototypical EAP, EapH1, relies on plasticity within a single inhibitory site to block the activities of CG and NE. However, whether other EAPs follow similar structure-function relationships is unclear. To address this question, we studied the inhibitory properties of the first (Eap1) and second (Eap2) domains of the modular extracellular adherence protein of S. aureus and determined their structures when bound to CG and NE, respectively. We observed that both Eap1 and Eap2 displayed time-dependent inhibition of CG (on the order of 10 -9 M) and of NE (on the order of 10 -10 M). We also found that whereas the structures of Eap1 and Eap2 bound to CG showed an overall inhibitory mode like that seen previously for EapH1, the structures of Eap1 and Eap2 bound to NE revealed a new inhibitory mode involving a distal region of the EAP domain. Using site-directed mutagenesis of Eap1 and Eap2, along with enzyme assays, we confirmed the roles of interfacial residues in NSP inhibition. Taken together, our work demonstrates that EAPs can form structurally divergent complexes with two closely related serine proteases and further suggests that certain EAPs may be capable of inhibiting two NSPs simultaneously.
- Department of Biochemistry & Molecular Biophysics, Kansas State University, Manhattan, Kansas, USA.
Organizational Affiliation: