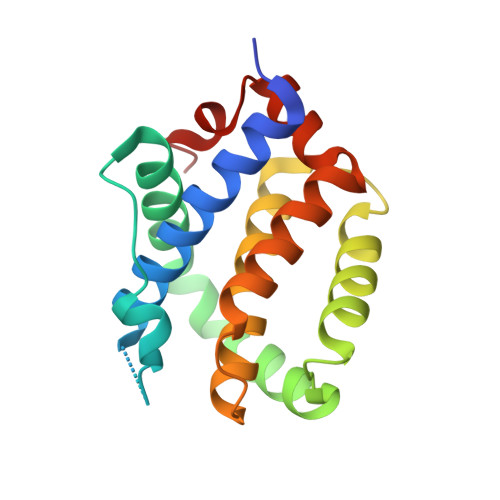
Peptides from human BNIP5 and PXT1 and non-native binders of pro-apoptotic BAK can directly activate or inhibit BAK-mediated membrane permeabilization.
Aguilar, F., Yu, S., Grant, R.A., Swanson, S., Ghose, D., Su, B.G., Sarosiek, K.A., Keating, A.E.(2023) Structure 31: 265-281.e7
- PubMed: 36706751
- DOI: https://doi.org/10.1016/j.str.2023.01.001
- Primary Citation of Related Structures:
8CZF, 8CZG, 8CZH - PubMed Abstract:
Apoptosis is important for development and tissue homeostasis, and its dysregulation can lead to diseases, including cancer. As an apoptotic effector, BAK undergoes conformational changes that promote mitochondrial outer membrane disruption, leading to cell death. This is termed "activation" and can be induced by peptides from the human proteins BID, BIM, and PUMA. To identify additional peptides that can regulate BAK, we used computational protein design, yeast surface display screening, and structure-based energy scoring to identify 10 diverse new binders. We discovered peptides from the human proteins BNIP5 and PXT1 and three non-native peptides that activate BAK in liposome assays and induce cytochrome c release from mitochondria. Crystal structures and binding studies reveal a high degree of similarity among peptide activators and inhibitors, ruling out a simple function-determining property. Our results shed light on the vast peptide sequence space that can regulate BAK function and will guide the design of BAK-modulating tools and therapeutics.
- Department of Biology, Massachusetts Institute of Technology, Cambridge, MA, USA.
Organizational Affiliation: