Expanding the Structural Diversity at the Phenylene Core of Ligands for the von Hippel-Lindau E3 Ubiquitin Ligase: Development of Highly Potent Hypoxia-Inducible Factor-1 alpha Stabilizers.
Vu, L.P., Diehl, C.J., Casement, R., Bond, A.G., Steinebach, C., Strasek, N., Bricelj, A., Perdih, A., Schnakenburg, G., Sosic, I., Ciulli, A., Gutschow, M.(2023) J Med Chem 66: 12776-12811
- PubMed: 37708384
- DOI: https://doi.org/10.1021/acs.jmedchem.3c00434
- Primary Citation of Related Structures:
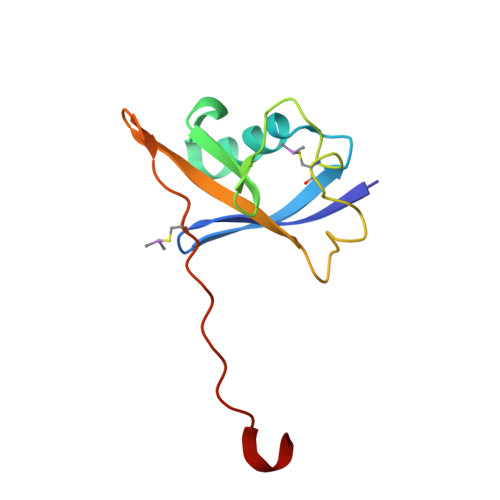
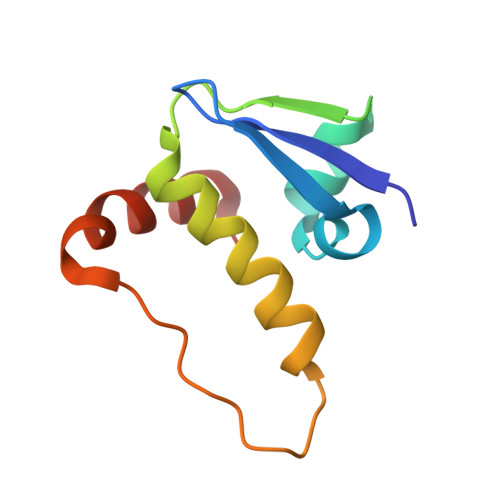
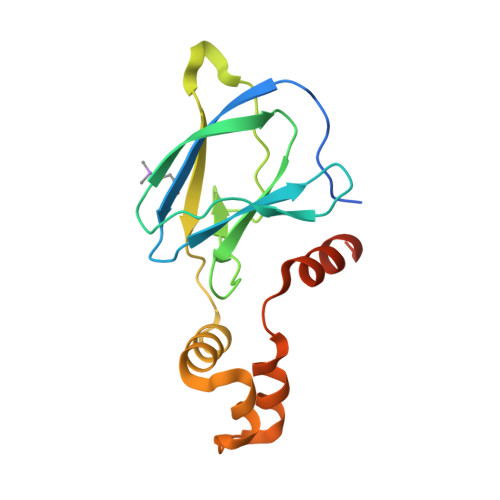
8CQE, 8CQK, 8CQL - PubMed Abstract:
Hypoxia-inducible factor-1α (HIF-1α) constitutes the principal mediator of cellular adaptation to hypoxia in humans. The HIF-1α protein level and activity are tightly regulated by the ubiquitin E3 ligase von Hippel-Lindau (VHL). Here, we performed a structure-guided and bioactivity-driven design of new VHL inhibitors. Our iterative and combinatorial strategy focused on chemical variability at the phenylene unit and encompassed further points of diversity. The exploitation of tailored phenylene fragments and the stereoselective installation of the benzylic methyl group provided potent VHL ligands. Three high-resolution structures of VHL-ligand complexes were determined, and bioactive conformations of these ligands were explored. The most potent inhibitor ( 30 ) exhibited dissociation constants lower than 40 nM, independently determined by fluorescence polarization and surface plasmon resonance and an enhanced cellular potency, as evidenced by its superior ability to induce HIF-1α transcriptional activity. Our work is anticipated to inspire future efforts toward HIF-1α stabilizers and new ligands for proteolysis-targeting chimera (PROTAC) degraders.
- Pharmaceutical Institute, Pharmaceutical & Medicinal Chemistry, University of Bonn, An der Immenburg 4, D-53121 Bonn, Germany.
Organizational Affiliation: