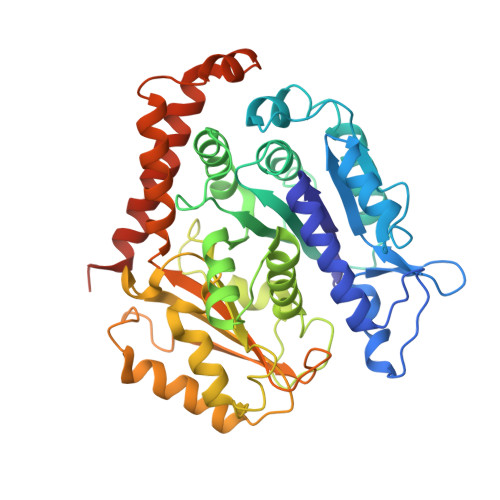
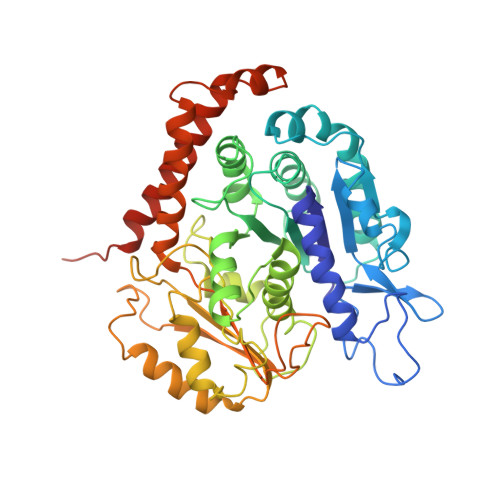
The mitotic role of adenomatous polyposis coli requires its bilateral interaction with tubulin and microtubules.
Serre, L., Delaroche, J., Vinit, A., Schoehn, G., Denarier, E., Fourest-Lieuvin, A., Arnal, I.(2023) J Cell Sci 136
- PubMed: 36541084
- DOI: https://doi.org/10.1242/jcs.260152
- Primary Citation of Related Structures:
8C5C - PubMed Abstract:
Adenomatous polyposis coli (APC) is a scaffold protein with tumour suppressor properties. Mutations causing the loss of its C-terminal domain (APC-C), which bears cytoskeleton-regulating sequences, correlate with colorectal cancer. The cellular roles of APC in mitosis are widely studied, but the molecular mechanisms of its interaction with the cytoskeleton are poorly understood. Here, we investigated how APC-C regulates microtubule properties, and found that it promotes both microtubule growth and shrinkage. Strikingly, APC-C accumulates at shrinking microtubule extremities, a common characteristic of depolymerases. Cryo-electron microscopy revealed that APC-C adopts an extended conformation along the protofilament crest and showed the presence of ring-like tubulin oligomers around the microtubule wall, which required the presence of two APC-C sub-domains. A mutant of APC-C that was incapable of decorating microtubules with ring-like tubulin oligomers exhibited a reduced effect on microtubule dynamics. Finally, whereas native APC-C rescued defective chromosome alignment in metaphase cells silenced for APC, the ring-incompetent mutant failed to correct mitotic defects. Thus, the bilateral interaction of APC-C with tubulin and microtubules likely contributes to its mitotic functions.
- Université Grenoble Alpes, Inserm U1216, CEA, CNRS, Grenoble Institut Neurosciences, GIN, 38000 Grenoble, France.
Organizational Affiliation: