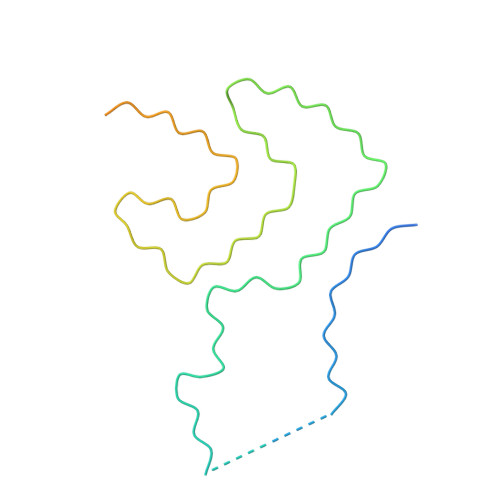
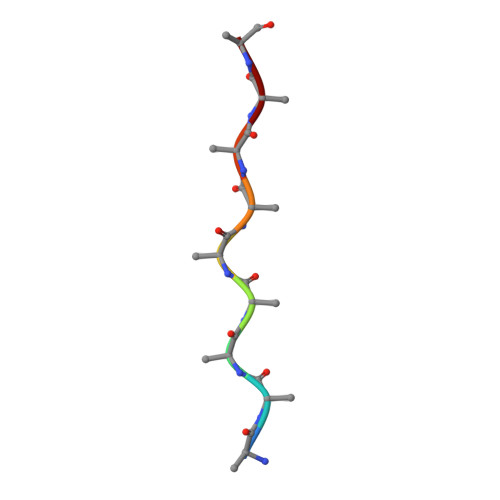
New SNCA mutation and structures of alpha-synuclein filaments from juvenile-onset synucleinopathy.
Yang, Y., Garringer, H.J., Shi, Y., Lovestam, S., Peak-Chew, S., Zhang, X., Kotecha, A., Bacioglu, M., Koto, A., Takao, M., Spillantini, M.G., Ghetti, B., Vidal, R., Murzin, A.G., Scheres, S.H.W., Goedert, M.(2023) Acta Neuropathol 145: 561-572
- PubMed: 36847833
- DOI: https://doi.org/10.1007/s00401-023-02550-8
- Primary Citation of Related Structures:
8BQV, 8BQW, 8CE7, 8CEB - PubMed Abstract:
A 21-nucleotide duplication in one allele of SNCA was identified in a previously described disease with abundant α-synuclein inclusions that we now call juvenile-onset synucleinopathy (JOS). This mutation translates into the insertion of MAAAEKT after residue 22 of α-synuclein, resulting in a protein of 147 amino acids. Both wild-type and mutant proteins were present in sarkosyl-insoluble material that was extracted from frontal cortex of the individual with JOS and examined by electron cryo-microscopy. The structures of JOS filaments, comprising either a single protofilament, or a pair of protofilaments, revealed a new α-synuclein fold that differs from the folds of Lewy body diseases and multiple system atrophy (MSA). The JOS fold consists of a compact core, the sequence of which (residues 36-100 of wild-type α-synuclein) is unaffected by the mutation, and two disconnected density islands (A and B) of mixed sequences. There is a non-proteinaceous cofactor bound between the core and island A. The JOS fold resembles the common substructure of MSA Type I and Type II dimeric filaments, with its core segment approximating the C-terminal body of MSA protofilaments B and its islands mimicking the N-terminal arm of MSA protofilaments A. The partial similarity of JOS and MSA folds extends to the locations of their cofactor-binding sites. In vitro assembly of recombinant wild-type α-synuclein, its insertion mutant and their mixture yielded structures that were distinct from those of JOS filaments. Our findings provide insight into a possible mechanism of JOS fibrillation in which mutant α-synuclein of 147 amino acids forms a nucleus with the JOS fold, around which wild-type and mutant proteins assemble during elongation.
- Medical Research Council Laboratory of Molecular Biology, Cambridge, UK.
Organizational Affiliation: