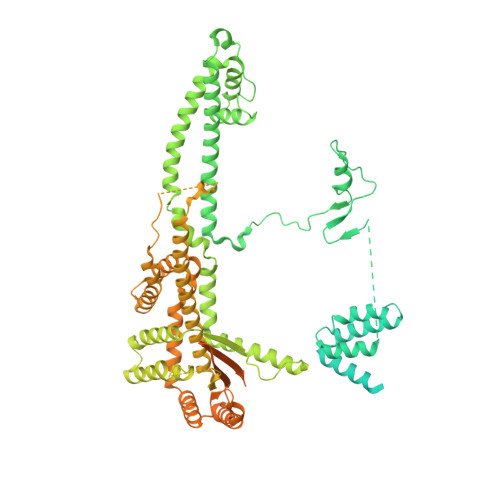
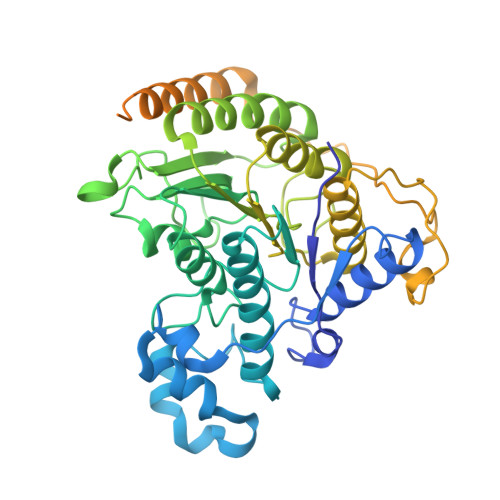
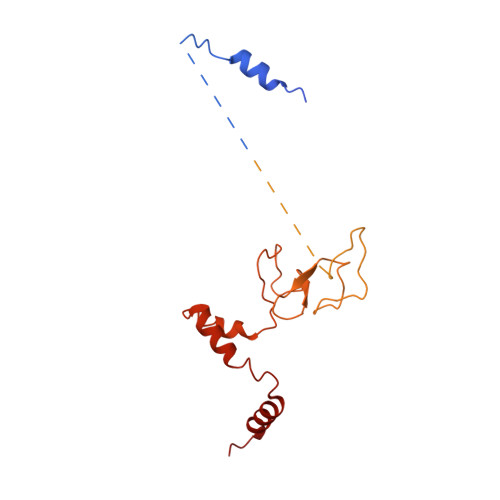
Mechanism of assembly, activation and lysine selection by the SIN3B histone deacetylase complex.
Wan, M.S.M., Muhammad, R., Koliopoulos, M.G., Roumeliotis, T.I., Choudhary, J.S., Alfieri, C.(2023) Nat Commun 14: 2556-2556
- PubMed: 37137925
- DOI: https://doi.org/10.1038/s41467-023-38276-0
- Primary Citation of Related Structures:
8BPA, 8BPB, 8BPC, 8C60 - PubMed Abstract:
Lysine acetylation in histone tails is a key post-translational modification that controls transcription activation. Histone deacetylase complexes remove histone acetylation, thereby repressing transcription and regulating the transcriptional output of each gene. Although these complexes are drug targets and crucial regulators of organismal physiology, their structure and mechanisms of action are largely unclear. Here, we present the structure of a complete human SIN3B histone deacetylase holo-complex with and without a substrate mimic. Remarkably, SIN3B encircles the deacetylase and contacts its allosteric basic patch thereby stimulating catalysis. A SIN3B loop inserts into the catalytic tunnel, rearranges to accommodate the acetyl-lysine moiety, and stabilises the substrate for specific deacetylation, which is guided by a substrate receptor subunit. Our findings provide a model of specificity for a main transcriptional regulator conserved from yeast to human and a resource of protein-protein interactions for future drug designs.
- Division of Structural Biology, Chester Beatty Laboratories, The Institute of Cancer Research, London, UK.
Organizational Affiliation: