Crystal structure of Fusobacterium nucleatum fusolisin protease
Isupov, M.N., Wiener, R., Rouvinski, A., Fahoum, J., Kumar, M., Read, R.J.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Biological assembly 1 assigned by authors.
Biological assembly 2 assigned by authors.
Biological assembly 3 assigned by authors.
Biological assembly 4 assigned by authors.
Macromolecule Content
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Fusolisin | A [auth AAA], B [auth BBB], C [auth CCC], D [auth DDD] | 585 | Fusobacterium nucleatum | Mutation(s): 0 | 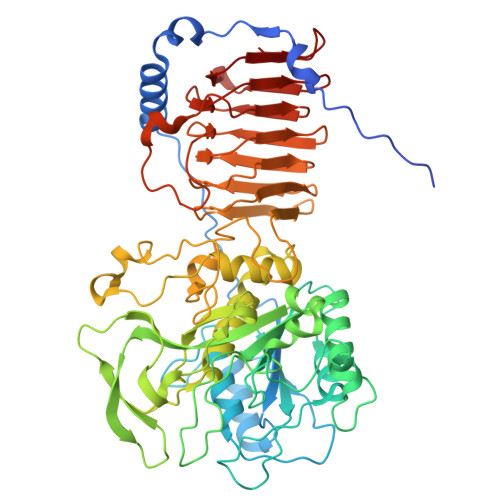 |
UniProt | |||||
Find proteins for A0A068B7P9 (Fusobacterium nucleatum subsp. nucleatum (strain ATCC 25586 / DSM 15643 / BCRC 10681 / CIP 101130 / JCM 8532 / KCTC 2640 / LMG 13131 / VPI 4355)) Explore A0A068B7P9 Go to UniProtKB: A0A068B7P9 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | A0A068B7P9 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 115.475 | α = 90 |
| b = 115.475 | β = 90 |
| c = 196.786 | γ = 90 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| DIALS | data reduction |
| pointless | data scaling |
| Aimless | data scaling |
| PHASER | phasing |
| PARROT | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Not funded | -- |
RCSB PDB Core Operations are funded by the U.S. National Science Foundation (DBI-2321666), the US Department of Energy (DE-SC0019749), and the National Cancer Institute, National Institute of Allergy and Infectious Diseases, and National Institute of General Medical Sciences of the National Institutes of Health under grant R01GM157729. RCSB PDB uses resources of the National Energy Research Scientific Computing Center (NERSC), a Department of Energy User Facility.




