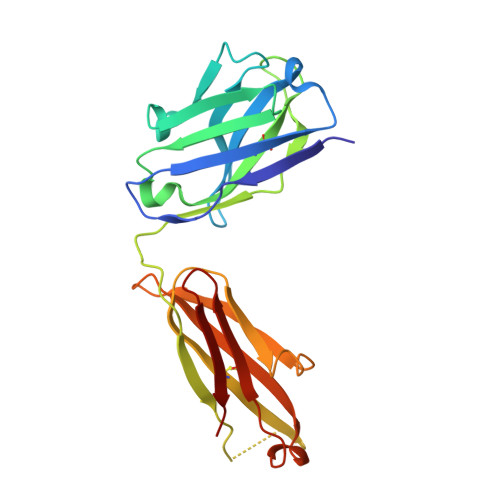
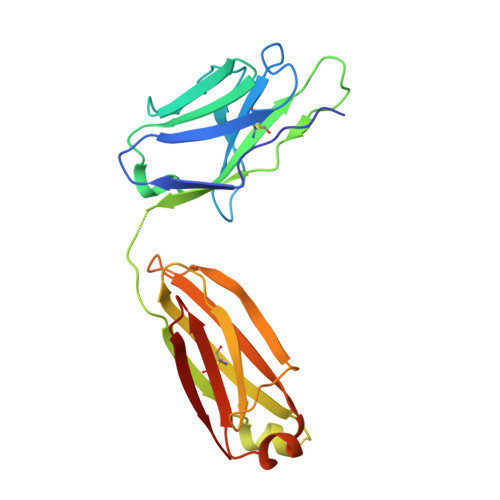
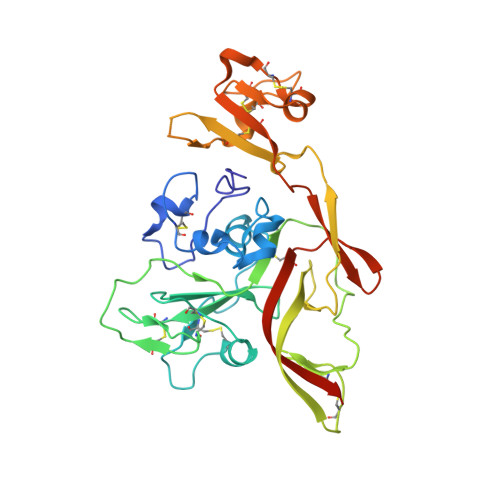
Multifunctional human monoclonal antibody combination mediates protection against Rift Valley fever virus at low doses.
Chapman, N.S., Hulswit, R.J.G., Westover, J.L.B., Stass, R., Paesen, G.C., Binshtein, E., Reidy, J.X., Engdahl, T.B., Handal, L.S., Flores, A., Gowen, B.B., Bowden, T.A., Crowe Jr., J.E.(2023) Nat Commun 14: 5650-5650
- PubMed: 37704627
- DOI: https://doi.org/10.1038/s41467-023-41171-3
- Primary Citation of Related Structures:
8AWL, 8AWM - PubMed Abstract:
The zoonotic Rift Valley fever virus (RVFV) can cause severe disease in humans and has pandemic potential, yet no approved vaccine or therapy exists. Here we describe a dual-mechanism human monoclonal antibody (mAb) combination against RVFV that is effective at minimal doses in a lethal mouse model of infection. We structurally analyze and characterize the binding mode of a prototypical potent Gn domain-A-binding antibody that blocks attachment and of an antibody that inhibits infection by abrogating the fusion process as previously determined. Surprisingly, the Gn domain-A antibody does not directly block RVFV Gn interaction with the host receptor low density lipoprotein receptor-related protein 1 (LRP1) as determined by a competitive assay. This study identifies a rationally designed combination of human mAbs deserving of future investigation for use in humans against RVFV infection. Using a two-pronged mechanistic approach, we demonstrate the potent efficacy of a rationally designed combination mAb therapeutic.
- Department of Pathology, Microbiology, and Immunology, Vanderbilt University Medical Center, Nashville, TN, 37232, USA.
Organizational Affiliation: