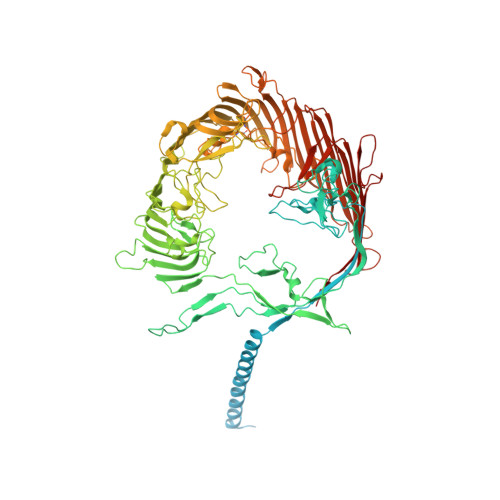
A multidomain connector links the outer membrane and cell wall in phylogenetically deep-branching bacteria.
von Kugelgen, A., van Dorst, S., Alva, V., Bharat, T.A.M.(2022) Proc Natl Acad Sci U S A 119: e2203156119-e2203156119
- PubMed: 35943982
- DOI: https://doi.org/10.1073/pnas.2203156119
- Primary Citation of Related Structures:
8AE1 - PubMed Abstract:
Deinococcus radiodurans is a phylogenetically deep-branching extremophilic bacterium that is remarkably tolerant to numerous environmental stresses, including large doses of ultraviolet (UV) radiation and extreme temperatures. It can even survive in outer space for several years. This endurance of D. radiodurans has been partly ascribed to its atypical cell envelope comprising an inner membrane, a large periplasmic space with a thick peptidoglycan (PG) layer, and an outer membrane (OM) covered by a surface layer (S-layer). Despite intense research, molecular principles governing envelope organization and OM stabilization are unclear in D. radiodurans and related bacteria. Here, we report a electron cryomicroscopy (cryo-EM) structure of the abundant D. radiodurans OM protein SlpA, showing how its C-terminal segment forms homotrimers of 30-stranded β-barrels in the OM, whereas its N-terminal segment forms long, homotrimeric coiled coils linking the OM to the PG layer via S-layer homology (SLH) domains. Furthermore, using protein structure prediction and sequence-based bioinformatic analysis, we show that SlpA-like putative OM-PG connector proteins are widespread in phylogenetically deep-branching Gram-negative bacteria. Finally, combining our atomic structures with fluorescence and electron microscopy of cell envelopes of wild-type and mutant bacterial strains, we report a model for the cell surface of D. radiodurans . Our results will have important implications for understanding the cell surface organization and hyperstability of D. radiodurans and related bacteria and the evolutionary transition between Gram-negative and Gram-positive bacteria.
- Structural Studies Division, MRC Laboratory of Molecular Biology, Cambridge CB2 0QH, United Kingdom.
Organizational Affiliation: