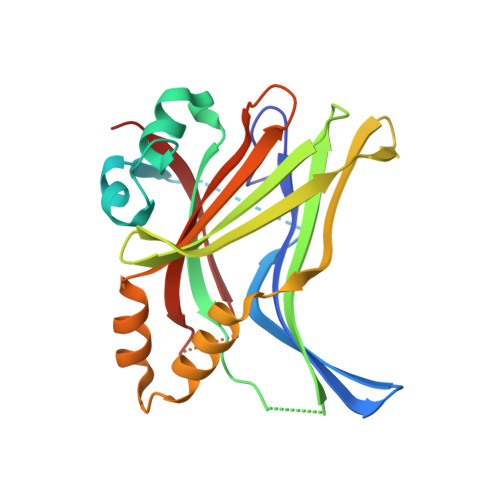
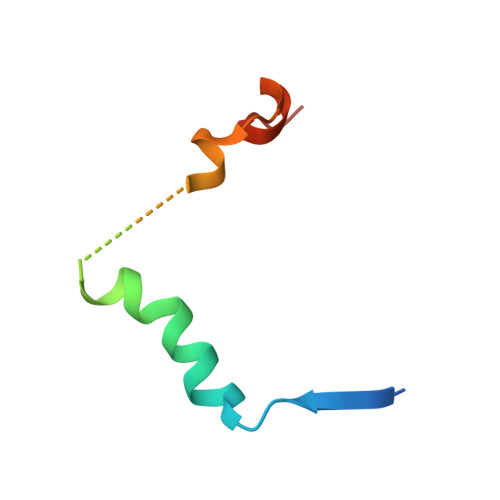
N-terminal beta-strand in YAP is critical for stronger binding to scalloped relative to TEAD transcription factor.
Fedir, B., Yannick, M., Marco, M., Patrizia, F., Catherine, Z., Frederic, V., Dirk, E., Joerg, K., Clemens, S., Camilo, V.V., Patrick, C.(2023) Protein Sci 32: e4545-e4545
- PubMed: 36522189
- DOI: https://doi.org/10.1002/pro.4545
- Primary Citation of Related Structures:
8A8Q, 8A8R - PubMed Abstract:
The yes-associated protein (YAP) regulates the transcriptional activity of the TEAD transcription factors that are key in the control of organ morphogenesis. YAP interacts with TEAD via three secondary structure elements: a β-strand, an α-helix, and an Ω-loop. Earlier results have shown that the β-strand has only a marginal contribution in the YAP:TEAD interaction, but we show here that it significantly enhances the affinity of YAP for the Drosophila homolog of TEAD, scalloped (Sd). Nuclear magnetic resonance shows that the β-strand adopts a more rigid conformation once bound to Sd; pre-steady state kinetic measurements show that the YAP:Sd complex is more stable. Although the crystal structures of the YAP:TEAD and YAP:Sd complexes reveal no differences at the binding interface that could explain these results. Molecular Dynamics simulations are in line with our experimental findings regarding β-strand stability and overall binding affinity of YAP to TEAD and Sd. In particular, RMSF, correlated motion and MMGBSA analyses suggest that β-sheet fluctuations play a relevant role in YAP 53-57 β-strand dissociation from TEAD4 and contribute to the lower affinity of YAP for TEAD4. Identifying a clear mechanism leading to the difference in YAP's β-strand stability proved to be challenging, pointing to the potential relevance of multiple modest structural changes or fluctuations for regulation of binding affinity.
- Disease Area Oncology, Novartis Institutes for Biomedical Research, Basel, Switzerland.
Organizational Affiliation: