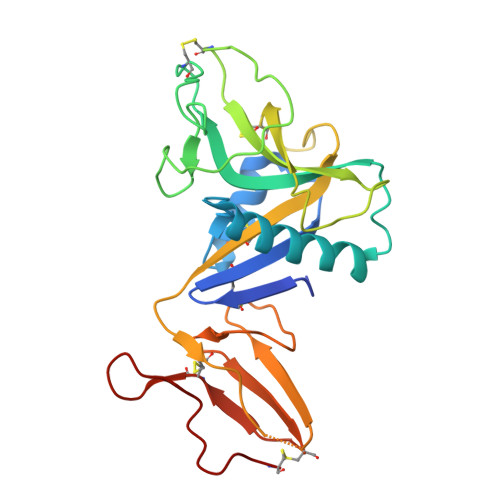
Dimerization of the C-type lectin-like receptor CD93 promotes its binding to Multimerin-2 in endothelial cells.
Barbera, S., Raucci, L., Tassone, G., Tinti, L., Prischi, F., Santucci, A., Mongiat, M., Tosi, G.M., Galvagni, F., Dimberg, A., Pozzi, C., Orlandini, M.(2023) Int J Biol Macromol 224: 453-464
- PubMed: 36265539
- DOI: https://doi.org/10.1016/j.ijbiomac.2022.10.136
- Primary Citation of Related Structures:
8A59 - PubMed Abstract:
Blocking the signaling activated by the plasma membrane receptor CD93 has recently been demonstrated a useful tool in antiangiogenic treatment and oncotherapy. In the proliferating endothelium, CD93 regulates cell adhesion, migration, and vascular maturation, yet it is unclear how CD93 interacts with the extracellular matrix activating signaling pathways involved in the vascular remodeling. Here for the first time we show that in endothelial cells CD93 is structured as a dimer and that this oligomeric form is physiologically instrumental for the binding of CD93 to its ligand Multimerin-2. Crystallographic X-ray analysis of recombinant CD93 reveals the crucial role played by the C-type lectin-like and sushi-like domains in arranging as an antiparallel dimer to achieve a functional binding state, providing key information for the future design of new drugs able to hamper CD93 function in neovascular pathologies.
- Department of Biotechnology, Chemistry and Pharmacy, University of Siena, Siena 53100, Italy; Department of Immunology, Genetics and Pathology, Science for Life Laboratory, Uppsala University, Rudbeck Laboratory, Uppsala SE-75185, Sweden.
Organizational Affiliation: