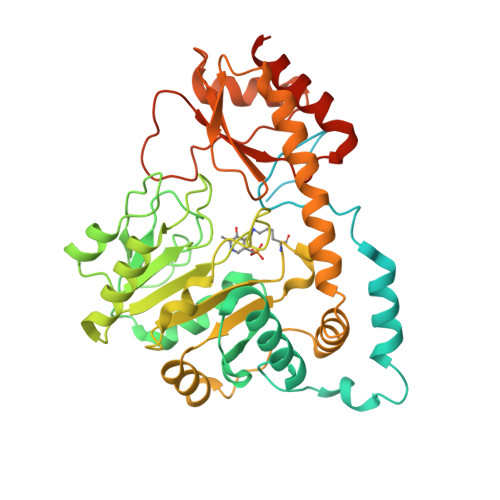
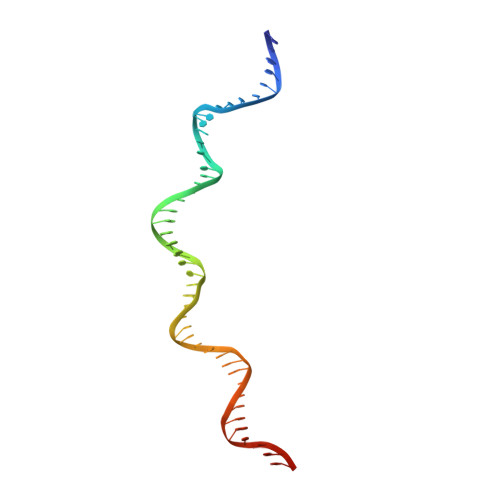
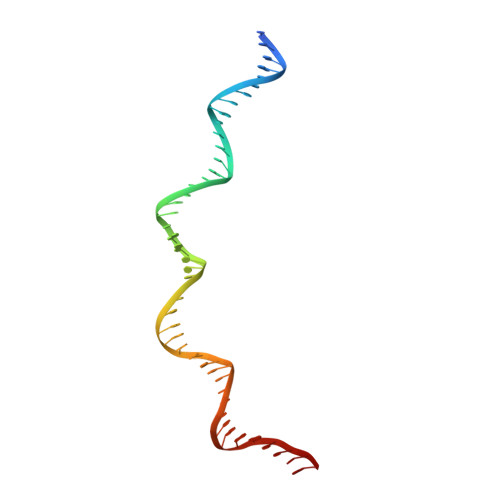
Structural insights into the DNA recognition mechanism by the bacterial transcription factor PdxR.
Freda, I., Exertier, C., Barile, A., Chaves-Sanjuan, A., Vega, M.V., Isupov, M.N., Harmer, N.J., Gugole, E., Swuec, P., Bolognesi, M., Scipioni, A., Savino, C., Di Salvo, M.L., Contestabile, R., Vallone, B., Tramonti, A., Montemiglio, L.C.(2023) Nucleic Acids Res 51: 8237-8254
- PubMed: 37378428
- DOI: https://doi.org/10.1093/nar/gkad552
- Primary Citation of Related Structures:
7PQ9, 7ZLA, 7ZN5, 7ZPA, 7ZTH - PubMed Abstract:
Specificity in protein-DNA recognition arises from the synergy of several factors that stem from the structural and chemical signatures encoded within the targeted DNA molecule. Here, we deciphered the nature of the interactions driving DNA recognition and binding by the bacterial transcription factor PdxR, a member of the MocR family responsible for the regulation of pyridoxal 5'-phosphate (PLP) biosynthesis. Single particle cryo-EM performed on the PLP-PdxR bound to its target DNA enabled the isolation of three conformers of the complex, which may be considered as snapshots of the binding process. Moreover, the resolution of an apo-PdxR crystallographic structure provided a detailed description of the transition of the effector domain to the holo-PdxR form triggered by the binding of the PLP effector molecule. Binding analyses of mutated DNA sequences using both wild type and PdxR variants revealed a central role of electrostatic interactions and of the intrinsic asymmetric bending of the DNA in allosterically guiding the holo-PdxR-DNA recognition process, from the first encounter through the fully bound state. Our results detail the structure and dynamics of the PdxR-DNA complex, clarifying the mechanism governing the DNA-binding mode of the holo-PdxR and the regulation features of the MocR family of transcription factors.
- Department of Biochemical Sciences "A. Rossi Fanelli", Sapienza, University of Rome, Rome 00185, Italy.
Organizational Affiliation: