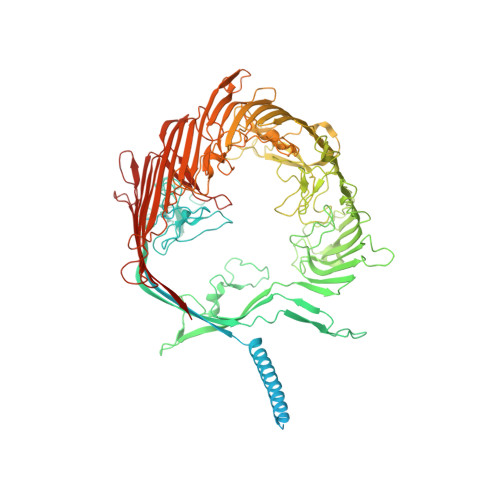
The cryo-EM structure of the S-layer deinoxanthin-binding complex of Deinococcus radiodurans informs properties of its environmental interactions.
Farci, D., Haniewicz, P., de Sanctis, D., Iesu, L., Kereiche, S., Winterhalter, M., Piano, D.(2022) J Biological Chem 298: 102031-102031
- PubMed: 35577074
- DOI: https://doi.org/10.1016/j.jbc.2022.102031
- Primary Citation of Related Structures:
7ZGX, 7ZGY - PubMed Abstract:
The radiation-resistant bacterium Deinococcus radiodurans is known as the world's toughest bacterium. The S-layer of D. radiodurans, consisting of several proteins on the surface of the cellular envelope and intimately associated with the outer membrane, has therefore been useful as a model for structural and functional studies. Its main proteinaceous unit, the S-layer deinoxanthin-binding complex (SDBC), is a hetero-oligomeric assembly known to contribute to the resistance against environmental stress and have porin functional features; however, its precise structure is unknown. Here, we resolved the structure of the SDBC at ∼2.5 Å resolution by cryo-EM and assigned the sequence of its main subunit, the protein DR_2577. This structure is characterized by a pore region, a massive β-barrel organization, a stalk region consisting of a trimeric coiled coil, and a collar region at the base of the stalk. We show that each monomer binds three Cu ions and one Fe ion and retains one deinoxanthin molecule and two phosphoglycolipids, all exclusive to D. radiodurans. Finally, electrophysiological characterization of the SDBC shows that it exhibits transport properties with several amino acids. Taken together, these results highlight the SDBC as a robust structure displaying both protection and sieving functions that facilitates exchanges with the environment.
- Department of Plant Physiology, Warsaw University of Life Sciences - SGGW, Warsaw, Poland; Department of Chemistry, Umeå University, Umeå, Sweden. Electronic address: domenica.farci@umu.se.
Organizational Affiliation: