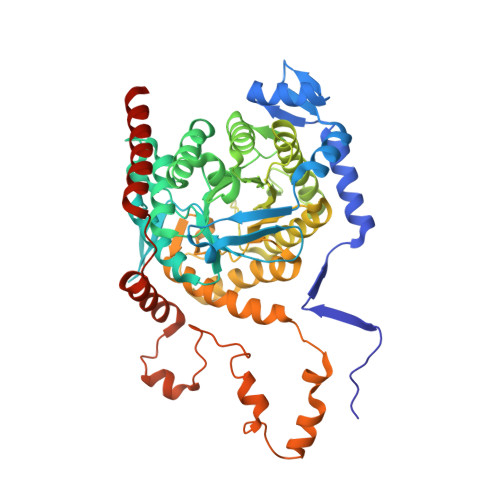
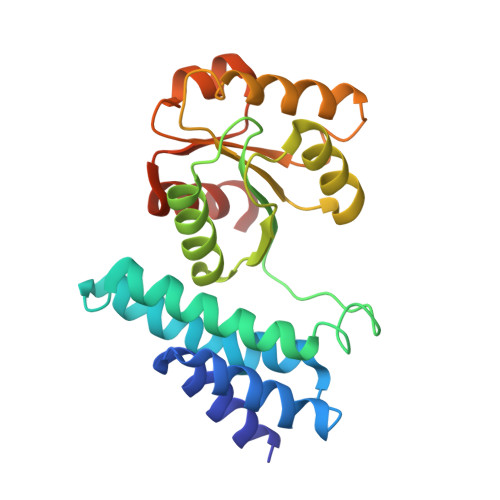
Insights into pyrrolysine function from structures of a trimethylamine methyltransferase and its corrinoid protein complex.
Li, J., Kang, P.T., Jiang, R., Lee, J.Y., Soares, J.A., Krzycki, J.A., Chan, M.K.(2023) Commun Biol 6: 54-54
- PubMed: 36646841
- DOI: https://doi.org/10.1038/s42003-022-04397-3
- Primary Citation of Related Structures:
7XCL, 7XCM, 7XCN - PubMed Abstract:
The 22nd genetically encoded amino acid, pyrrolysine, plays a unique role in the key step in the growth of methanogens on mono-, di-, and tri-methylamines by activating the methyl group of these substrates for transfer to a corrinoid cofactor. Previous crystal structures of the Methanosarcina barkeri monomethylamine methyltransferase elucidated the structure of pyrrolysine and provide insight into its role in monomethylamine activation. Herein, we report the second structure of a pyrrolysine-containing protein, the M. barkeri trimethylamine methyltransferase MttB, and its structure bound to sulfite, a substrate analog of trimethylamine. We also report the structure of MttB in complex with its cognate corrinoid protein MttC, which specifically receives the methyl group from the pyrrolysine-activated trimethylamine substrate during methanogenesis. Together these structures provide key insights into the role of pyrrolysine in methyl group transfer from trimethylamine to the corrinoid cofactor in MttC.
- School of Life Sciences, and Center of Novel Biomaterials, The Chinese University of Hong Kong, Shatin, Hong Kong, China.
Organizational Affiliation: