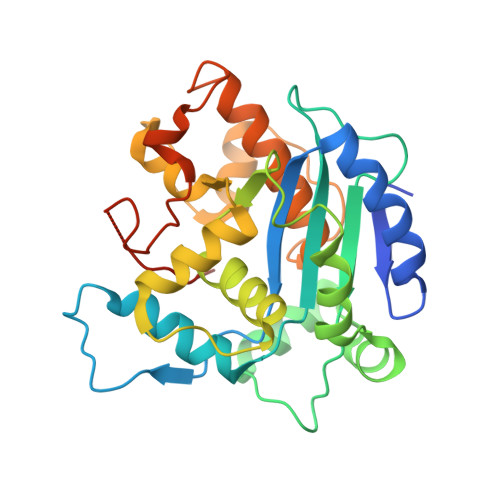
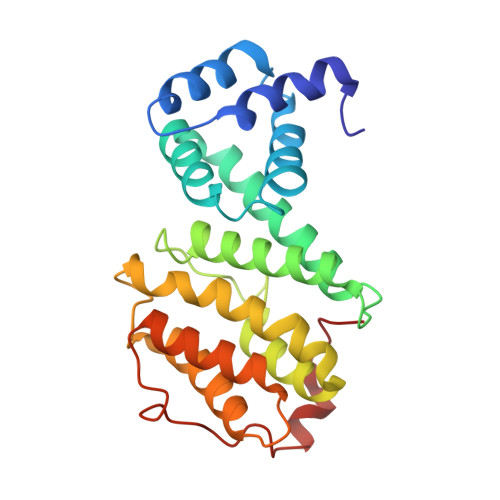
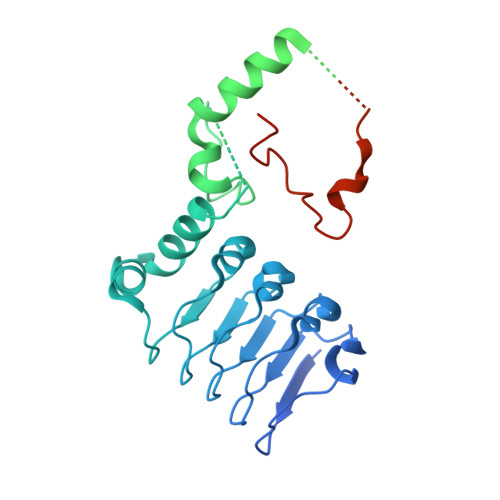
Structure of the human Ccr4-Not nuclease module using X-ray crystallography and electron paramagnetic resonance spectroscopy distance measurements.
Zhang, Q., Pavanello, L., Potapov, A., Bartlam, M., Winkler, G.S.(2022) Protein Sci 31: 758-764
- PubMed: 34923703
- DOI: https://doi.org/10.1002/pro.4262
- Primary Citation of Related Structures:
7VOI - PubMed Abstract:
Regulated degradation of mature, cytoplasmic mRNA is a key step in eukaryotic gene regulation. This process is typically initiated by the recruitment of deadenylase enzymes by cis-acting elements in the 3' untranslated region resulting in the shortening and removal of the 3' poly(A) tail of the target mRNA. The Ccr4-Not complex, a major eukaryotic deadenylase, contains two exoribonuclease subunits with selectivity toward poly(A): Caf1 and Ccr4. The Caf1 deadenylase subunit binds the MIF4G domain of the large subunit CNOT1 (Not1) that is the scaffold of the complex. The Ccr4 nuclease is connected to the complex via its leucine-rich repeat (LRR) domain, which binds Caf1, whereas the catalytic activity of Ccr4 is provided by its EEP domain. While the relative positions of the MIF4G domain of CNOT1, the Caf1 subunit, and the LRR domain of Ccr4 are clearly defined in current models, the position of the EEP nuclease domain of Ccr4 is ambiguous. Here, we use X-ray crystallography, the AlphaFold resource of predicted protein structures, and pulse electron paramagnetic resonance spectroscopy to determine and validate the position of the EEP nuclease domain of Ccr4 resulting in an improved model of the human Ccr4-Not nuclease module.
- Nankai International Advanced Research Institute (Shenzhen Futian), College of Life Sciences, State Key Laboratory of Medicinal Chemical Biology, Nankai University, Tianjin, China.
Organizational Affiliation: