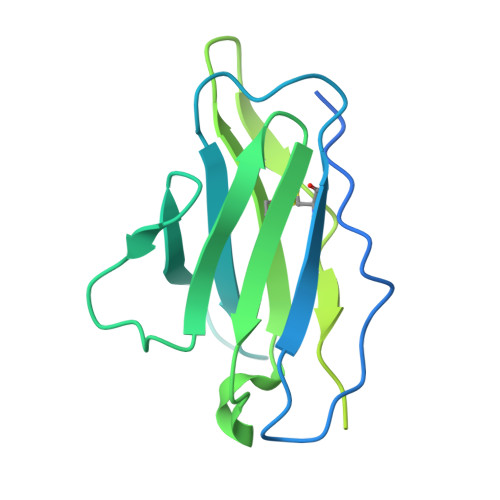
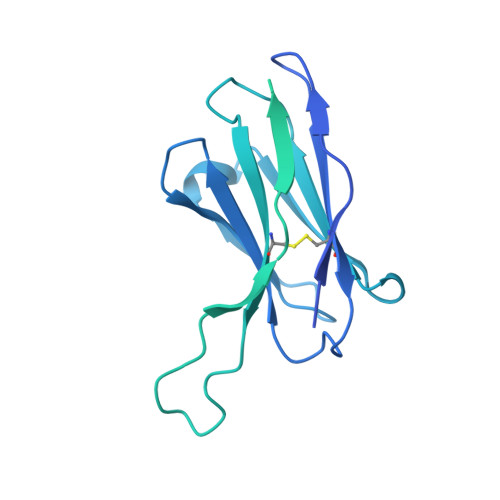
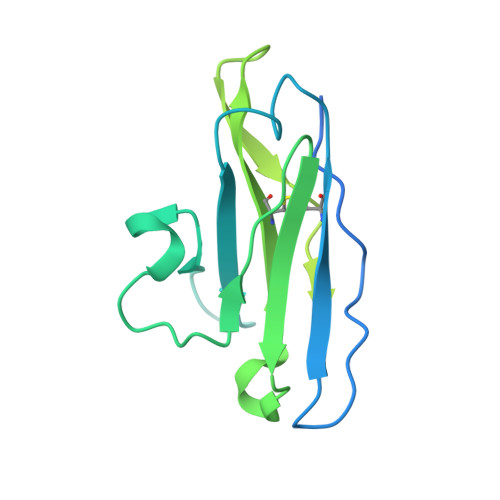
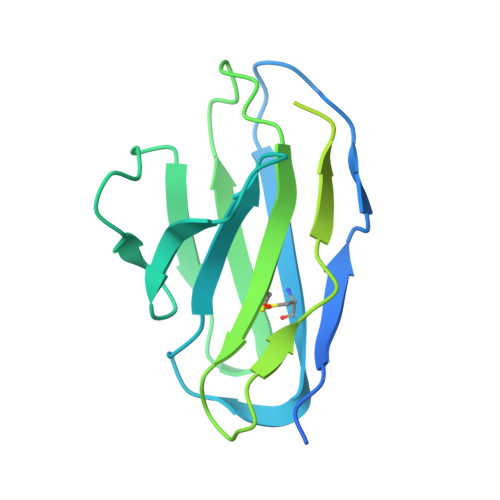
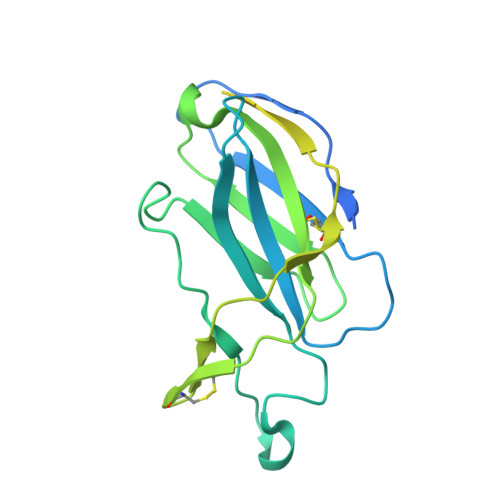
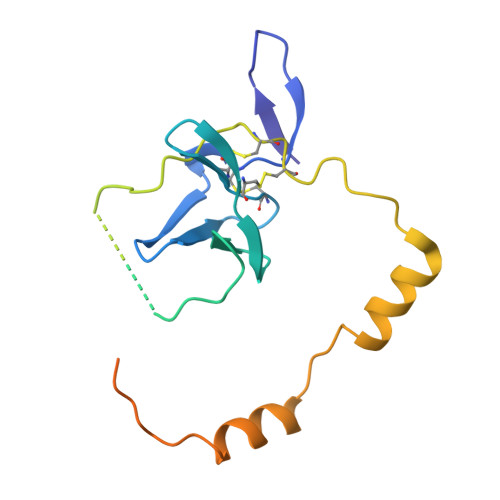
Structure of the hepatitis C virus E1E2 glycoprotein complex.
Torrents de la Pena, A., Sliepen, K., Eshun-Wilson, L., Newby, M.L., Allen, J.D., Zon, I., Koekkoek, S., Chumbe, A., Crispin, M., Schinkel, J., Lander, G.C., Sanders, R.W., Ward, A.B.(2022) Science 378: 263-269
- PubMed: 36264808
- DOI: https://doi.org/10.1126/science.abn9884
- Primary Citation of Related Structures:
7T6X - PubMed Abstract:
Hepatitis C virus (HCV) infection is a leading cause of chronic liver disease, cirrhosis, and hepatocellular carcinoma in humans and afflicts more than 58 million people worldwide. The HCV envelope E1 and E2 glycoproteins are essential for viral entry and comprise the primary antigenic target for neutralizing antibody responses. The molecular mechanisms of E1E2 assembly, as well as how the E1E2 heterodimer binds broadly neutralizing antibodies, remain elusive. Here, we present the cryo-electron microscopy structure of the membrane-extracted full-length E1E2 heterodimer in complex with three broadly neutralizing antibodies-AR4A, AT1209, and IGH505-at ~3.5-angstrom resolution. We resolve the interface between the E1 and E2 ectodomains and deliver a blueprint for the rational design of vaccine immunogens and antiviral drugs.
- Department of Integrative Structural Biology and Computational Biology, The Scripps Research Institute, La Jolla, CA 92037, USA.
Organizational Affiliation: