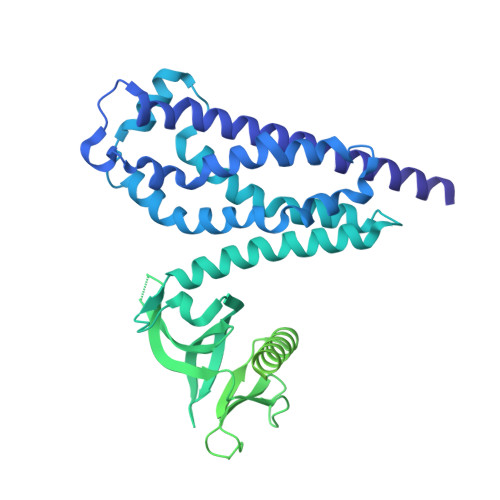
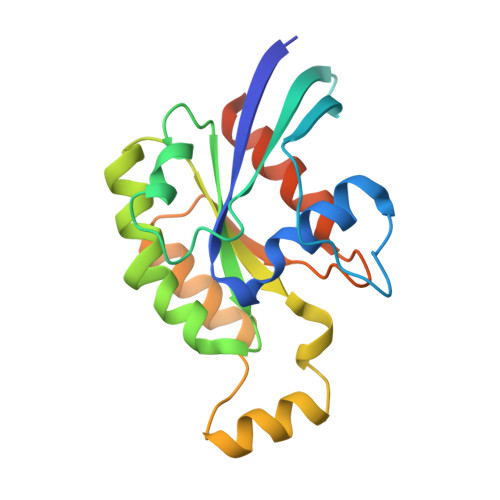
Structural/functional studies of Trio provide insights into its configuration and show that conserved linker elements enhance its activity for Rac1.
Bandekar, S.J., Chen, C.L., Ravala, S.K., Cash, J.N., Avramova, L.V., Zhalnina, M.V., Gutkind, J.S., Li, S., Tesmer, J.J.G.(2022) J Biological Chem 298: 102209-102209
- PubMed: 35779635
- DOI: https://doi.org/10.1016/j.jbc.2022.102209
- Primary Citation of Related Structures:
7SJ4 - PubMed Abstract:
Trio is a large and highly conserved metazoan signaling scaffold that contains two Dbl family guanine nucleotide exchange factor (GEF) modules, TrioN and TrioC, selective for Rac and RhoA GTPases, respectively. The GEF activities of TrioN and TrioC are implicated in several cancers, especially uveal melanoma. However, little is known about how these modules operate in the context of larger fragments of Trio. Here we show via negative stain electron microscopy that the N-terminal region of Trio is extended and could thus serve as a rigid spacer between the N-terminal putative lipid-binding domain and TrioN, whereas the C-terminal half of Trio seems globular. We found that regions C-terminal to TrioN enhance its Rac1 GEF activity and thus could play a regulatory role. We went on to characterize a minimal, well-behaved Trio fragment with enhanced activity, Trio 1284 - 1959 , in complex with Rac1 using cryo-electron microscopy and hydrogen-deuterium exchange mass spectrometry and found that the region conferring enhanced activity is disordered. Deletion of two different strongly conserved motifs in this region eliminated this enhancement, suggesting that they form transient intramolecular interactions that promote GEF activity. Because Dbl family RhoGEF modules have been challenging to directly target with small molecules, characterization of accessory Trio domains such as these may provide alternate routes for the development of therapeutics that inhibit Trio activity in human cancer.
- Department of Medicinal Chemistry, University of Michigan, Ann Arbor, Michigan, USA; Life Sciences Institute, University of Michigan, Ann Arbor, Michigan, USA.
Organizational Affiliation: