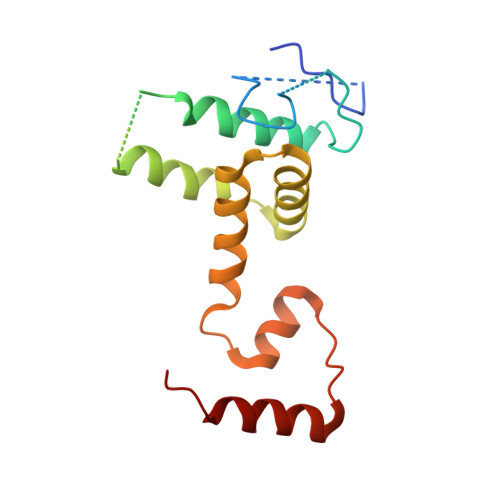
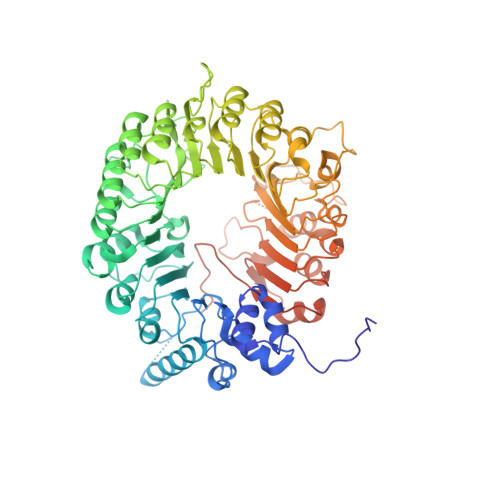
A conformational switch in the SCF-D3/MAX2 ubiquitin ligase facilitates strigolactone signalling.
Tal, L., Palayam, M., Ron, M., Young, A., Britt, A., Shabek, N.(2022) Nat Plants 8: 561-573
- PubMed: 35484202
- DOI: https://doi.org/10.1038/s41477-022-01145-7
- Primary Citation of Related Structures:
7SA1 - PubMed Abstract:
Strigolactones (SLs) are a class of plant hormones that regulate numerous processes of growth and development. SL perception and signal activation involves interaction between F-box E3 ubiquitin ligase D3/MAX2 and DWARF14 (D14) α/β-hydrolase in a SL-dependent manner and targeting of D53/SMXL6/7/8 transcriptional repressors (SMXLs) for proteasome-mediated degradation. D3/MAX2 has been shown to exist in multiple conformational states in which the C-terminal helix (CTH) undergoes a closed-to-open dynamics and regulates D14 binding and SL perception. Despite the multiple modes of D3-D14 interactions found in vitro, the residues that regulate the conformational switch of D3/MAX2 CTH in targeting D53/SMXLs and the subsequent effect on SL signalling remain unclear. Here we elucidate the functional dynamics of ASK1-D3/MAX2 in SL signalling by leveraging conformational switch mutants in vitro and in plants. We report the crystal structure of a dislodged CTH of the ASK1-D3 mutant and demonstrate that disruptions in CTH plasticity via either CRISPR-Cas9 genome editing or expression of point mutation mutants result in impairment of SL signalling. We show that the conformational switch in ASK1-D3/MAX2 CTH directly regulates ubiquitin-mediated protein degradation. A dislodged conformation involved in D53/SMXLs SL-dependent recruitment and ubiquitination and an engaged conformation are required for the release of polyubiquitinated D53/SMXLs and subsequently D14 for proteasomal degradation. Finally, we uncovered an organic acid metabolite that can directly trigger the D3/MAX2 CTH conformational switch. Our findings unravel a new regulatory function of a SKP1-CUL1-F-box ubiquitin ligase in plant signalling.
- Department of Plant Biology, College of Biological Sciences, University of California - Davis, Davis, CA, USA.
Organizational Affiliation: