Broad betacoronavirus neutralization by a stem helix-specific human antibody.
Pinto, D., Sauer, M.M., Czudnochowski, N., Low, J.S., Tortorici, M.A., Housley, M.P., Noack, J., Walls, A.C., Bowen, J.E., Guarino, B., Rosen, L.E., di Iulio, J., Jerak, J., Kaiser, H., Islam, S., Jaconi, S., Sprugasci, N., Culap, K., Abdelnabi, R., Foo, C., Coelmont, L., Bartha, I., Bianchi, S., Silacci-Fregni, C., Bassi, J., Marzi, R., Vetti, E., Cassotta, A., Ceschi, A., Ferrari, P., Cippa, P.E., Giannini, O., Ceruti, S., Garzoni, C., Riva, A., Benigni, F., Cameroni, E., Piccoli, L., Pizzuto, M.S., Smithey, M., Hong, D., Telenti, A., Lempp, F.A., Neyts, J., Havenar-Daughton, C., Lanzavecchia, A., Sallusto, F., Snell, G., Virgin, H.W., Beltramello, M., Corti, D., Veesler, D.(2021) Science 373: 1109-1116
- PubMed: 34344823
- DOI: https://doi.org/10.1126/science.abj3321
- Primary Citation of Related Structures:
7RNJ - PubMed Abstract:
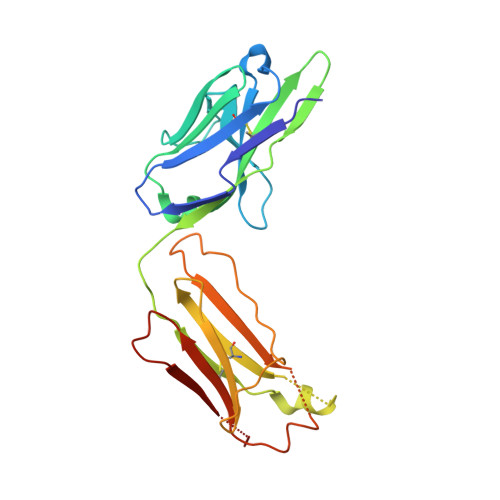
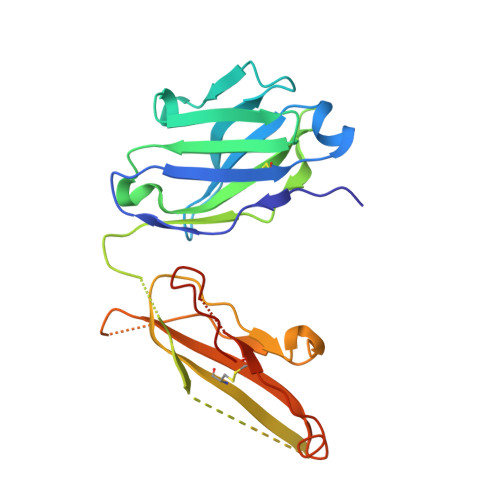
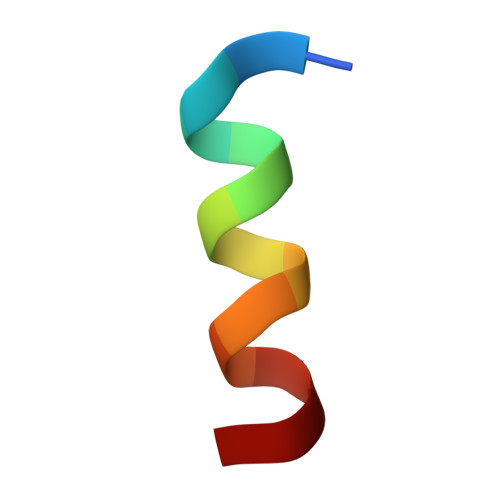
The spillovers of betacoronaviruses in humans and the emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants highlight the need for broad coronavirus countermeasures. We describe five monoclonal antibodies (mAbs) cross-reacting with the stem helix of multiple betacoronavirus spike glycoproteins isolated from COVID-19 convalescent individuals. Using structural and functional studies, we show that the mAb with the greatest breadth (S2P6) neutralizes pseudotyped viruses from three different subgenera through the inhibition of membrane fusion, and we delineate the molecular basis for its cross-reactivity. S2P6 reduces viral burden in hamsters challenged with SARS-CoV-2 through viral neutralization and Fc-mediated effector functions. Stem helix antibodies are rare, oftentimes of narrow specificity, and can acquire neutralization breadth through somatic mutations. These data provide a framework for structure-guided design of pan-betacoronavirus vaccines eliciting broad protection.
- Humabs Biomed SA, a subsidiary of Vir Biotechnology, 6500 Bellinzona, Switzerland.
Organizational Affiliation: