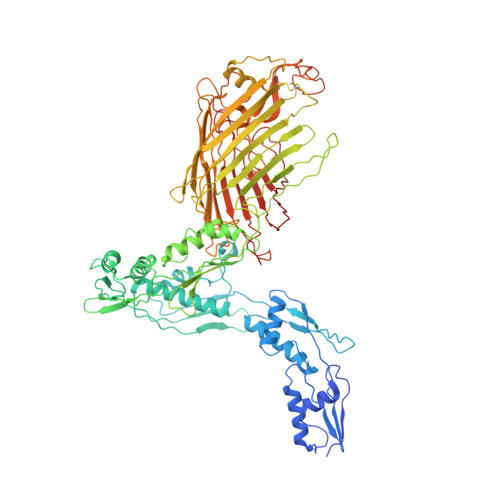
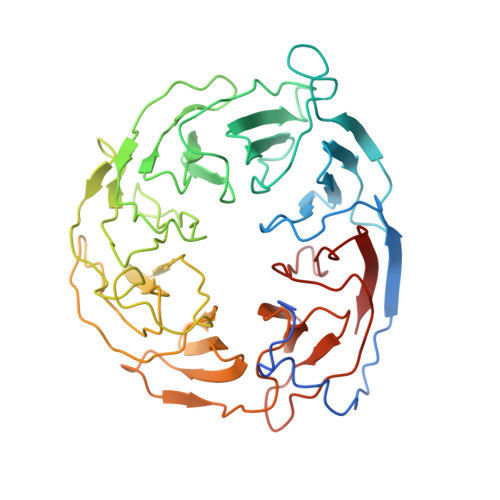
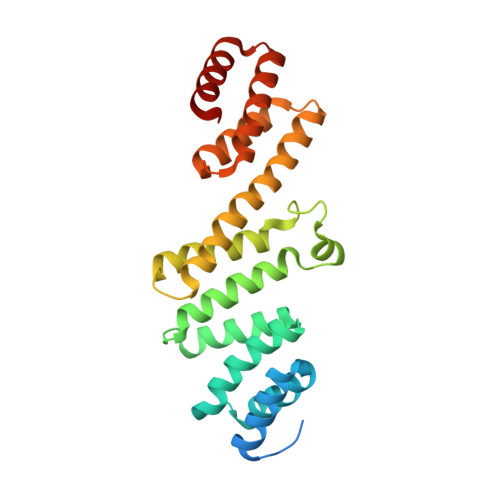
Plasticity within the barrel domain of BamA mediates a hybrid-barrel mechanism by BAM.
Wu, R., Bakelar, J.W., Lundquist, K., Zhang, Z., Kuo, K.M., Ryoo, D., Pang, Y.T., Sun, C., White, T., Klose, T., Jiang, W., Gumbart, J.C., Noinaj, N.(2021) Nat Commun 12: 7131-7131
- PubMed: 34880256
- DOI: https://doi.org/10.1038/s41467-021-27449-4
- Primary Citation of Related Structures:
7RI4, 7RI5, 7RI6, 7RI7, 7RI8, 7RI9, 7RJ5 - PubMed Abstract:
In Gram-negative bacteria, the biogenesis of β-barrel outer membrane proteins is mediated by the β-barrel assembly machinery (BAM). The mechanism employed by BAM is complex and so far- incompletely understood. Here, we report the structures of BAM in nanodiscs, prepared using polar lipids and native membranes, where we observe an outward-open state. Mutations in the barrel domain of BamA reveal that plasticity in BAM is essential, particularly along the lateral seam of the barrel domain, which is further supported by molecular dynamics simulations that show conformational dynamics in BAM are modulated by the accessory proteins. We also report the structure of BAM in complex with EspP, which reveals an early folding intermediate where EspP threads from the underside of BAM and incorporates into the barrel domain of BamA, supporting a hybrid-barrel budding mechanism in which the substrate is folded into the membrane sequentially rather than as a single unit.
- Interdisciplinary Life Science - PULSe, Purdue University, West Lafayette, IN, 47907, USA.
Organizational Affiliation: