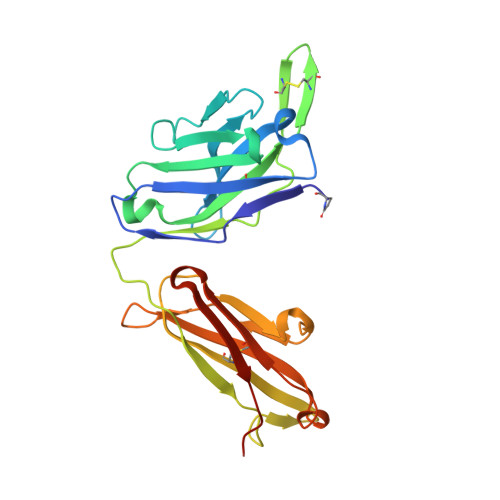
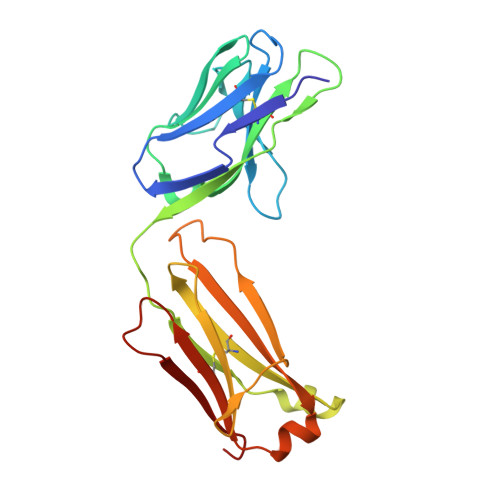
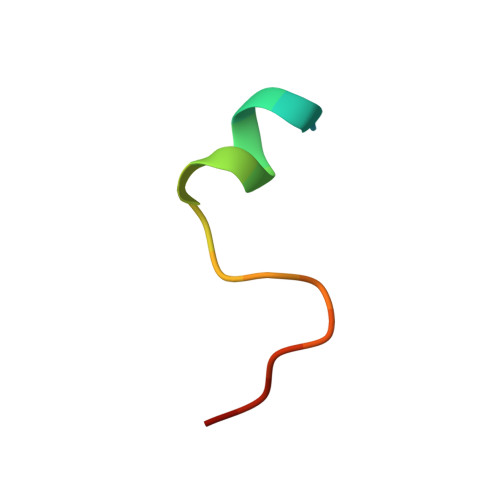
Structural definition of a pan-sarbecovirus neutralizing epitope on the spike S2 subunit.
Hurlburt, N.K., Homad, L.J., Sinha, I., Jennewein, M.F., MacCamy, A.J., Wan, Y.H., Boonyaratanakornkit, J., Sholukh, A.M., Jackson, A.M., Zhou, P., Burton, D.R., Andrabi, R., Ozorowski, G., Ward, A.B., Stamatatos, L., Pancera, M., McGuire, A.T.(2022) Commun Biol 5: 342-342
- PubMed: 35411021
- DOI: https://doi.org/10.1038/s42003-022-03262-7
- Primary Citation of Related Structures:
7RAQ - PubMed Abstract:
Three betacoronaviruses have crossed the species barrier and established human-to-human transmission causing significant morbidity and mortality in the past 20 years. The most current and widespread of these is SARS-CoV-2. The identification of CoVs with zoonotic potential in animal reservoirs suggests that additional outbreaks could occur. Monoclonal antibodies targeting conserved neutralizing epitopes on diverse CoVs can form the basis for prophylaxis and therapeutic treatments and enable the design of vaccines aimed at providing pan-CoV protection. We previously identified a neutralizing monoclonal antibody, CV3-25 that binds to the SARS-CoV-2 spike, neutralizes the SARS-CoV-2 Beta variant comparably to the ancestral Wuhan Hu-1 strain, cross neutralizes SARS-CoV-1 and binds to recombinant proteins derived from the spike-ectodomains of HCoV-OC43 and HCoV-HKU1. Here, we show that the neutralizing activity of CV3-25 is maintained against the Alpha, Delta, Gamma and Omicron variants of concern as well as a SARS-CoV-like bat coronavirus with zoonotic potential by binding to a conserved linear peptide in the stem-helix region. Negative stain electron microscopy and a 1.74 Å crystal structure of a CV3-25/peptide complex demonstrates that CV3-25 binds to the base of the stem helix at the HR2 boundary to an epitope that is distinct from other stem-helix directed neutralizing mAbs.
- Fred Hutchinson Cancer Research Center, Vaccine and Infectious Disease Division, Seattle, WA, USA.
Organizational Affiliation: