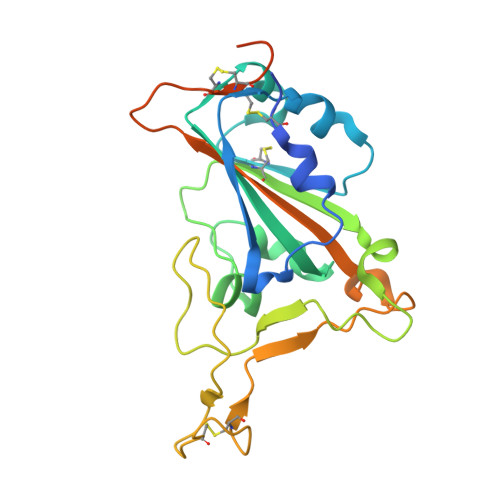
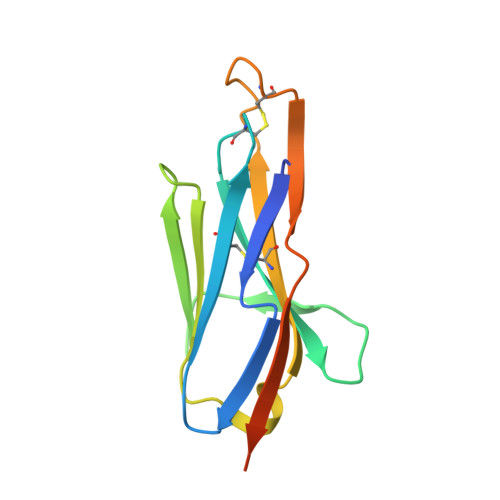
A Class of Shark-Derived Single-Domain Antibodies can Broadly Neutralize SARS-Related Coronaviruses and the Structural Basis of Neutralization and Omicron Escape.
Feng, B., Chen, Z., Sun, J., Xu, T., Wang, Q., Yi, H., Niu, X., Zhu, J., Fan, M., Hou, R., Shao, Y., Huang, S., Li, C., Hu, P., Zheng, P., He, P., Luo, J., Yan, Q., Xiong, X., Liu, J., Zhao, J., Chen, L.(2022) Small Methods 6: e2200387-e2200387
- PubMed: 35583124
- DOI: https://doi.org/10.1002/smtd.202200387
- Primary Citation of Related Structures:
7FBJ, 7FBK - PubMed Abstract:
The identification of a novel class of shark-derived single domain antibodies, named vnarbodies that show picomolar affinities binding to the receptor binding domain (RBD) of Wuhan and Alpha, Beta, Kappa, Delta, Delta-plus, and Lambda variants, is reported. Vnarbody 20G6 and 17F6 have broad neutralizing activities against all these SARS-CoV-2 viruses as well as other sarbecoviruses, including Pangolin coronavirus and Bat coronavirus. Intranasal administration of 20G6 effectively protects mice from the challenges of SARS-CoV-2 Wuhan and Beta variants. 20G6 and 17F6 contain a unique "WXGY" motif in the complementary determining region 3 that binds to a hidden epitope on RBD, which is highly conserved in sarbecoviruses through a novel β-sheet interaction. It is found that the S375F mutation on Omicron RBD disrupts the structure of β-strand, thus impair the binding with 20G6. The study demonstrates that shark-derived vnarbodies offer a prophylactic and therapeutic option against most SARS-CoV-2 variants and provide insights into antibody evasion by the Omicron variant.
- State Key Laboratory of Respiratory Disease, Institute of Infectious Disease, Guangzhou 8th People's Hospital & The First Affiliated Hospital of Guangzhou Medical University, Guangzhou, China.
Organizational Affiliation: