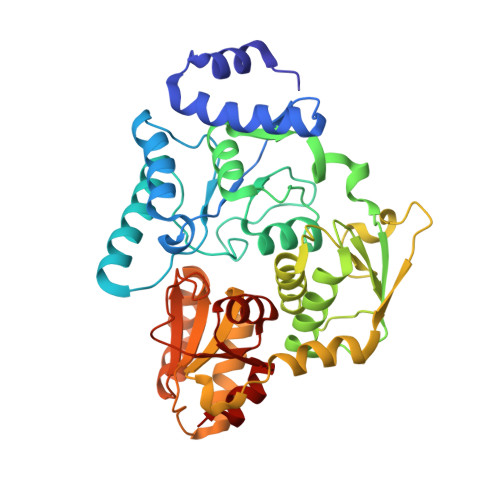
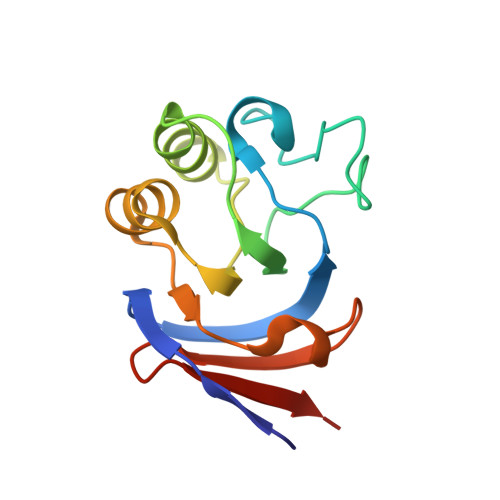
Crystal structures of aconitase X enzymes from bacteria and archaea provide insights into the molecular evolution of the aconitase superfamily.
Watanabe, S., Murase, Y., Watanabe, Y., Sakurai, Y., Tajima, K.(2021) Commun Biol 4: 687-687
- PubMed: 34099860
- DOI: https://doi.org/10.1038/s42003-021-02147-5
- Primary Citation of Related Structures:
7CNP, 7CNQ, 7CNR, 7CNS, 7D2R - PubMed Abstract:
Aconitase superfamily members catalyze the homologous isomerization of specific substrates by sequential dehydration and hydration and contain a [4Fe-4S] cluster. However, monomeric and heterodimeric types of function unknown aconitase X (AcnX) have recently been characterized as a cis-3-hydroxy-L-proline dehydratase (AcnX Type-I ) and mevalonate 5-phosphate dehydratase (AcnX Type-II ), respectively. We herein elucidated the crystal structures of AcnX Type-I from Agrobacterium tumefaciens (AtAcnX) and AcnX Type-II from Thermococcus kodakarensis (TkAcnX) without a ligand and in complex with substrates. AtAcnX and TkAcnX contained the [2Fe-2S] and [3Fe-4S] clusters, respectively, conforming to UV and EPR spectroscopy analyses. The binding sites of the [Fe-S] cluster and substrate were clearlydifferent from those that were completely conserved in other aconitase enzymes; however, theoverall structural frameworks and locations of active sites were partially similar to each other.These results provide novel insights into the evolutionary scenario of the aconitase superfamilybased on the recruitment hypothesis.
- Department of Bioscience, Graduate School of Agriculture, Ehime University, Matsuyama, Ehime, Japan. irab@agr.ehime-u.ac.jp.
Organizational Affiliation: