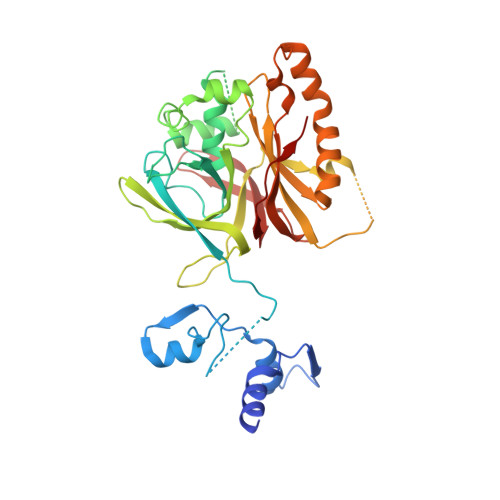
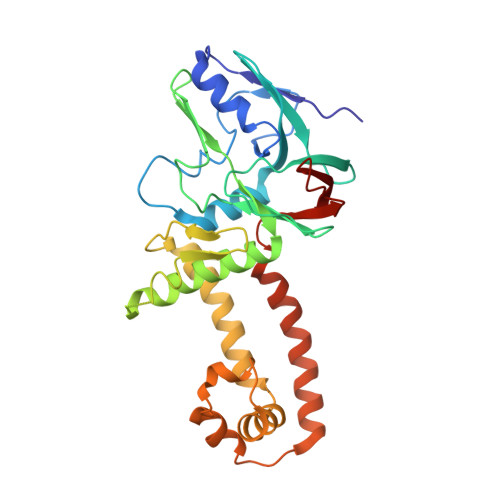
CryoEM structure of the tegumented capsid of Epstein-Barr virus.
Li, Z., Zhang, X., Dong, L., Pang, J., Xu, M., Zhong, Q., Zeng, M.S., Yu, X.(2020) Cell Res 30: 873-884
- PubMed: 32620850
- DOI: https://doi.org/10.1038/s41422-020-0363-0
- Primary Citation of Related Structures:
7BQT, 7BQX, 7BR7, 7BR8, 7BSI - PubMed Abstract:
Epstein-Barr virus (EBV) is the primary cause of infectious mononucleosis and has been shown to be closely associated with various malignancies. Here, we present a complete atomic model of EBV, including the icosahedral capsid, the dodecameric portal and the capsid-associated tegument complex (CATC). Our in situ portal from the tegumented capsid adopts a closed conformation with its channel valve holding the terminal viral DNA and with its crown region firmly engaged by three layers of ring-like dsDNA, which, together with the penton flexibility, effectively alleviates the capsid inner pressure placed on the portal cap. In contrast, the CATCs, through binding to the flexible penton vertices in a stoichiometric manner, accurately increase the inner capsid pressure to facilitate the pressure-driven genome delivery. Together, our results provide important insights into the mechanism by which the EBV capsid, portal, packaged genome and the CATCs coordinately achieve a pressure balance to simultaneously benefit both viral genome retention and ejection.
- Cryo-Electron Microscopy Research Center, The CAS Key Laboratory of Receptor Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai, 201203, China.
Organizational Affiliation: