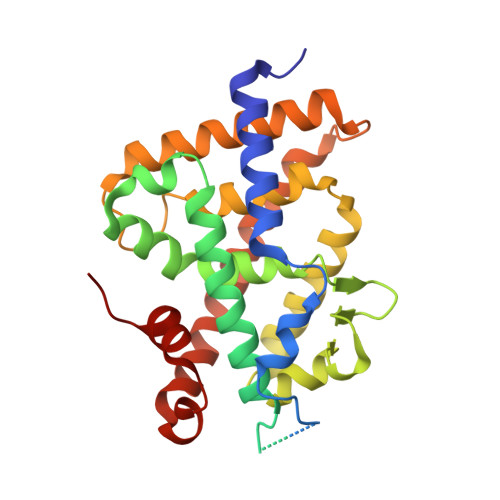
Design, Synthesis, Evaluation and Structure of Allenic 1 alpha ,25-Dihydroxyvitamin D 3 Analogs with Locked Mobility at C-17.
Fraga, R., Len, K., Lutzing, R., Laverny, G., Loureiro, J., Maestro, M.A., Rochel, N., Rodriguez-Borges, E., Mourino, A.(2021) Chemistry 27: 13384-13389
- PubMed: 34224173
- DOI: https://doi.org/10.1002/chem.202101578
- Primary Citation of Related Structures:
7B39 - PubMed Abstract:
Vitamin D receptor ligands have potential for the treatment of hyperproliferative diseases and disorders related to the immune system. However, hypercalcemic effects limit their therapeutical uses and call for the development of tissue-selective new analogs. We have designed and synthesized the first examples of 1α,25-dihydroxyvitamin D 3 analogs bearing an allenic unit attached to the D ring to restrict the side-chain conformational mobility. The triene system was constructed by a Pd 0 -mediated cyclization/Suzuki-Miyaura cross-coupling process in the presence of an allenic side chain. The allenic moiety was built through an orthoester-Claisen rearrangement of a propargylic alcohol. The biological activity and structure of (22S)-1α,25-dihydroxy-17,20-dien-24-homo-21-nor-vitamin D 3 bound to binding domain of the vitamin D receptor, provide information concerning side-chain conformational requirements for biological activity.
- Departamento de Química Orgánica, Laboratorio de Investigación Ignacio Ribas, Universidad de Santiago, Avda Ciencias s/n, 15782, Santiago de Compostela, Spain.
Organizational Affiliation: