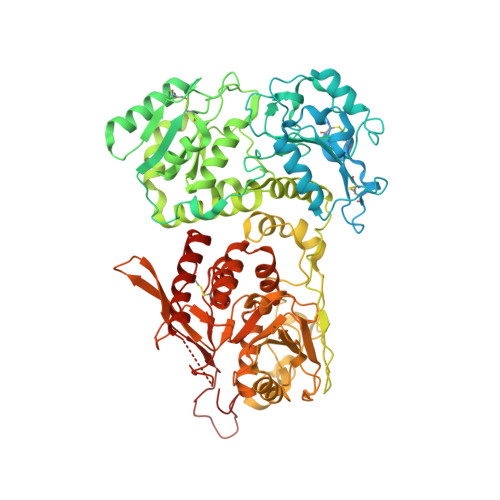
The structure of EXTL3 helps to explain the different roles of bi-domain exostosins in heparan sulfate synthesis.
Wilson, L.F.L., Dendooven, T., Hardwick, S.W., Echevarria-Poza, A., Tryfona, T., Krogh, K.B.R.M., Chirgadze, D.Y., Luisi, B.F., Logan, D.T., Mani, K., Dupree, P.(2022) Nat Commun 13: 3314-3314
- PubMed: 35676258
- DOI: https://doi.org/10.1038/s41467-022-31048-2
- Primary Citation of Related Structures:
7AU2, 7AUA - PubMed Abstract:
Heparan sulfate is a highly modified O-linked glycan that performs diverse physiological roles in animal tissues. Though quickly modified, it is initially synthesised as a polysaccharide of alternating β-D-glucuronosyl and N-acetyl-α-D-glucosaminyl residues by exostosins. These enzymes generally possess two glycosyltransferase domains (GT47 and GT64)-each thought to add one type of monosaccharide unit to the backbone. Although previous structures of murine exostosin-like 2 (EXTL2) provide insight into the GT64 domain, the rest of the bi-domain architecture is yet to be characterised; hence, how the two domains co-operate is unknown. Here, we report the structure of human exostosin-like 3 (EXTL3) in apo and UDP-bound forms. We explain the ineffectiveness of EXTL3's GT47 domain to transfer β-D-glucuronosyl units, and we observe that, in general, the bi-domain architecture would preclude a processive mechanism of backbone extension. We therefore propose that heparan sulfate backbone polymerisation occurs by a simple dissociative mechanism.
- Department of Biochemistry, University of Cambridge, Cambridge, CB2 1QW, UK.
Organizational Affiliation: