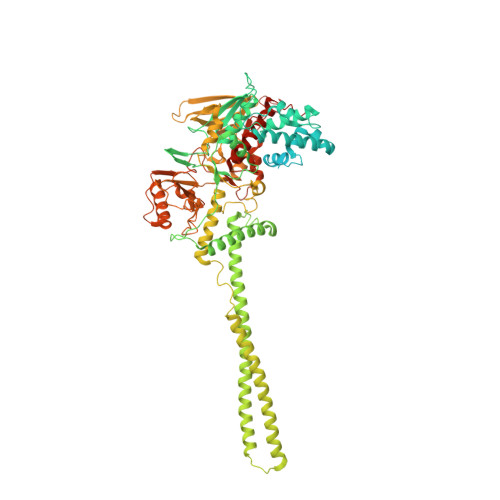
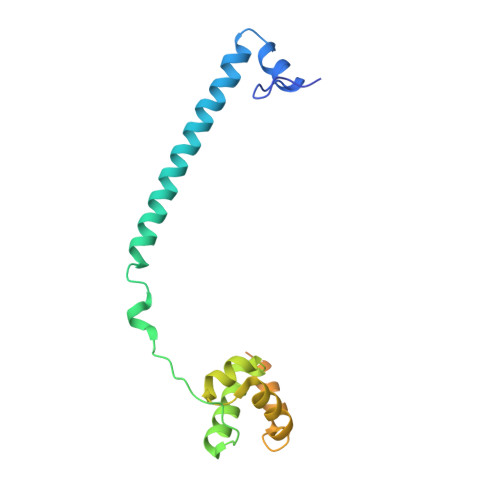
Fine-tuned KDM1A alternative splicing regulates human cardiomyogenesis through an enzymatic-independent mechanism.
Astro, V., Ramirez-Calderon, G., Pennucci, R., Caroli, J., Saera-Vila, A., Cardona-Londono, K., Forastieri, C., Fiacco, E., Maksoud, F., Alowaysi, M., Sogne, E., Falqui, A., Gonzalez, F., Montserrat, N., Battaglioli, E., Mattevi, A., Adamo, A.(2022) iScience 25: 104665-104665
- PubMed: 35856020
- DOI: https://doi.org/10.1016/j.isci.2022.104665
- Primary Citation of Related Structures:
7ZRY - PubMed Abstract:
The histone demethylase KDM1A is a multi-faceted regulator of vital developmental processes, including mesodermal and cardiac tube formation during gastrulation. However, it is unknown whether the fine-tuning of KDM1A splicing isoforms, already shown to regulate neuronal maturation, is crucial for the specification and maintenance of cell identity during cardiogenesis. Here, we discovered a temporal modulation of ubKDM1A and KDM1A+2a during human and mice fetal cardiac development and evaluated their impact on the regulation of cardiac differentiation. We revealed a severely impaired cardiac differentiation in KDM1A -/- hESCs that can be rescued by re-expressing ubKDM1A or catalytically impaired ubKDM1A-K661A, but not by KDM1A+2a or KDM1A+2a-K661A. Conversely, KDM1A+2a -/- hESCs give rise to functional cardiac cells, displaying increased beating amplitude and frequency and enhanced expression of critical cardiogenic markers. Our findings prove the existence of a divergent scaffolding role of KDM1A splice variants, independent of their enzymatic activity, during hESC differentiation into cardiac cells.
- Biological and Environmental Science and Engineering Division, King Abdullah University of Science and Technology, Thuwal 23955-6900, Saudi Arabia.
Organizational Affiliation: