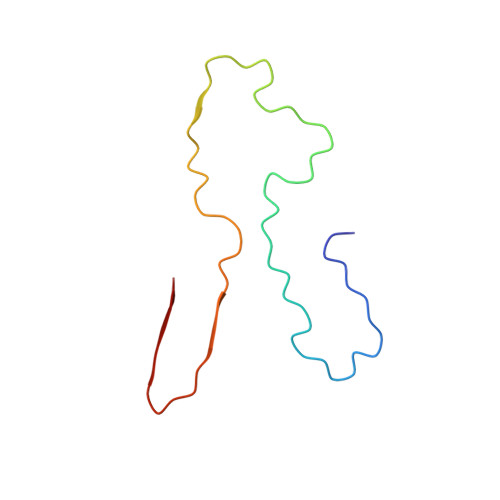
Amyloid fibril structure from the vascular variant of systemic AA amyloidosis.
Banerjee, S., Baur, J., Daniel, C., Pfeiffer, P.B., Hitzenberger, M., Kuhn, L., Wiese, S., Bijzet, J., Haupt, C., Amann, K.U., Zacharias, M., Hazenberg, B.P.C., Westermark, G.T., Schmidt, M., Fandrich, M.(2022) Nat Commun 13: 7261-7261
- PubMed: 36433936
- DOI: https://doi.org/10.1038/s41467-022-34636-4
- Primary Citation of Related Structures:
7ZKY - PubMed Abstract:
Systemic AA amyloidosis is a debilitating protein misfolding disease in humans and animals. In humans, it occurs in two variants that are called 'vascular' and 'glomerular', depending on the main amyloid deposition site in the kidneys. Using cryo electron microscopy, we here show the amyloid fibril structure underlying the vascular disease variant. Fibrils purified from the tissue of such patients are mainly left-hand twisted and contain two non-equal stacks of fibril proteins. They contrast in these properties to the fibrils from the glomerular disease variant which are right-hand twisted and consist of two structurally equal stacks of fibril proteins. Our data demonstrate that the different disease variants in systemic AA amyloidosis are associated with different fibril morphologies.
- Institute of Protein Biochemistry, Ulm University, 89081, Ulm, Germany.
Organizational Affiliation: