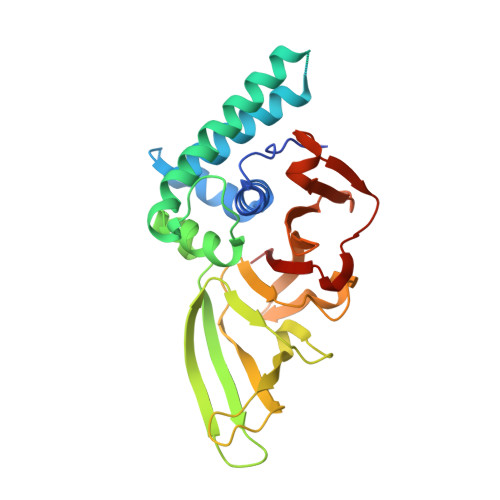
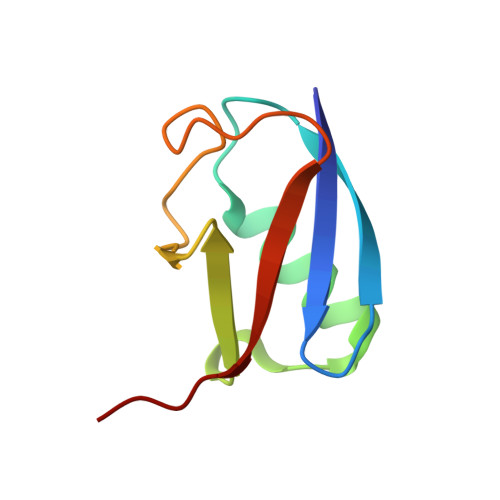
Native Semisynthesis of Isopeptide-Linked Substrates for Specificity Analysis of Deubiquitinases and Ubl Proteases.
Zhao, Z., O'Dea, R., Wendrich, K., Kazi, N., Gersch, M.(2023) J Am Chem Soc 145: 20801-20812
- PubMed: 37712884
- DOI: https://doi.org/10.1021/jacs.3c04062
- Primary Citation of Related Structures:
7ZJU, 7ZJV - PubMed Abstract:
Post-translational modifications with ubiquitin (Ub) and ubiquitin-like proteins (Ubls) are regulated by isopeptidases termed deubiquitinases (DUBs) and Ubl proteases. Here, we describe a mild chemical method for the preparation of fluorescence polarization substrates for these enzymes that is based on the activation of C-terminal Ub/Ubl hydrazides to acyl azides and their subsequent functionalization to isopeptides. The procedure is complemented by native purification routes and thus circumvents the previous need for desulfurization and refolding. Its broad applicability was demonstrated by the generation of fully cleavable substrates for Ub, SUMO1, SUMO2, NEDD8, ISG15, and Fubi. We employed these reagents for the investigation of substrate specificities of human UCHL3, USPL1, USP2, USP7, USP16, USP18, and USP36. Pronounced selectivity of USPL1 for SUMO2/3 over SUMO1 was observed, which we rationalize with crystal structures and biochemical assays, revealing a SUMO paralogue specificity mechanism distinct from SENP family deSUMOylases. Moreover, we investigated the recently identified Fubi proteases USP16 and USP36 and found both to act as bona fide deFubiylases, harboring catalytic activity against isopeptide-linked Fubi. Surprisingly, we also noticed the activity of both enzymes toward ISG15, previously not identified in chemoproteomics, which makes USP16 and USP36 the first human DUBs with specific isopeptidase activity toward three distinct modifiers. The methods described here for the preparation of isopeptide-linked, fully folded substrates will aid in the characterization of further DUBs/Ubl proteases. More broadly, our findings highlight possible limitations associated with fluorogenic substrates and Ubl activity-based probes and stress the importance of isopeptide-containing reagents for validating isopeptidase activities and quantifying substrate specificities.
- Chemical Genomics Centre, Max Planck Institute of Molecular Physiology, Otto-Hahn-Str. 15, 44227 Dortmund, Germany.
Organizational Affiliation: