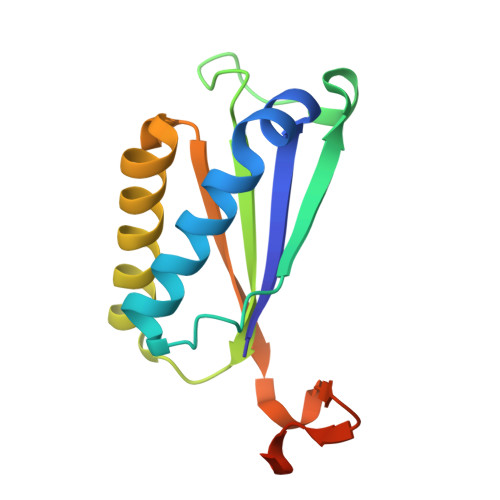
The structure of a tautomerase superfamily member linked to the type VI secretion system of Acinetobacter baumannii.
Pankov, G., Mol Avelar, G., Buchanan, G., Coulthurst, S.J., Hunter, W.N.(2023) Acta Crystallogr F Struct Biol Commun 79: 8-16
- PubMed: 36598351
- DOI: https://doi.org/10.1107/S2053230X22011414
- Primary Citation of Related Structures:
7YXV - PubMed Abstract:
Bacteria exploit specialized secretion systems to assist in competition for resources, in collaboration and in communication. Here, a protocol for the recombinant production, purification and crystallization of a protein linked to the Acinetobacter baumannii type VI secretion system is provided. A high-resolution structure of this trimeric protein is reported, revealing the characteristic dual β-α-β subunit fold typical of longer subunit members of the tautomerase superfamily. The protein does not appear to be toxic to bacteria or yeast under the conditions tested. The possible biological role of this protein is discussed.
- Division of Biological Chemistry and Drug Discovery, School of Life Sciences, University of Dundee, Dundee DD1 5EH, United Kingdom.
Organizational Affiliation: