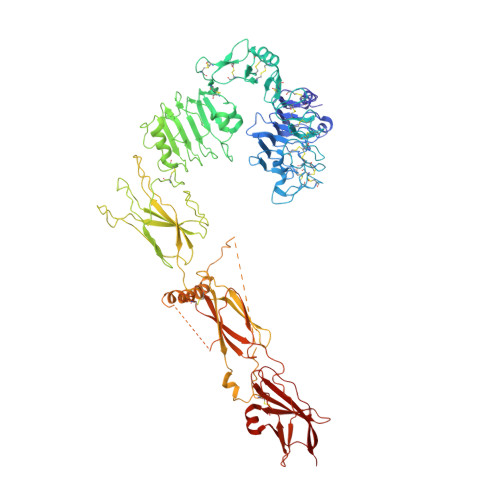
Functional selectivity of insulin receptor revealed by aptamer-trapped receptor structures.
Kim, J., Yunn, N.O., Park, M., Kim, J., Park, S., Kim, Y., Noh, J., Ryu, S.H., Cho, Y.(2022) Nat Commun 13: 6500-6500
- PubMed: 36310231
- DOI: https://doi.org/10.1038/s41467-022-34292-8
- Primary Citation of Related Structures:
7YQ3, 7YQ4, 7YQ5, 7YQ6, 8GUY - PubMed Abstract:
Activation of insulin receptor (IR) initiates a cascade of conformational changes and autophosphorylation events. Herein, we determined three structures of IR trapped by aptamers using cryo-electron microscopy. The A62 agonist aptamer selectively activates metabolic signaling. In the absence of insulin, the two A62 aptamer agonists of IR adopt an insulin-accessible arrowhead conformation by mimicking site-1/site-2' insulin coordination. Insulin binding at one site triggers conformational changes in one protomer, but this movement is blocked in the other protomer by A62 at the opposite site. A62 binding captures two unique conformations of IR with a similar stalk arrangement, which underlie Tyr1150 mono-phosphorylation (m-pY1150) and selective activation for metabolic signaling. The A43 aptamer, a positive allosteric modulator, binds at the opposite side of the insulin-binding module, and stabilizes the single insulin-bound IR structure that brings two FnIII-3 regions into closer proximity for full activation. Our results suggest that spatial proximity of the two FnIII-3 ends is important for m-pY1150, but multi-phosphorylation of IR requires additional conformational rearrangement of intracellular domains mediated by coordination between extracellular and transmembrane domains.
- Department of Life Sciences, Pohang University of Science and Technology (POSTECH), Pohang, 37673, Republic of Korea.
Organizational Affiliation: