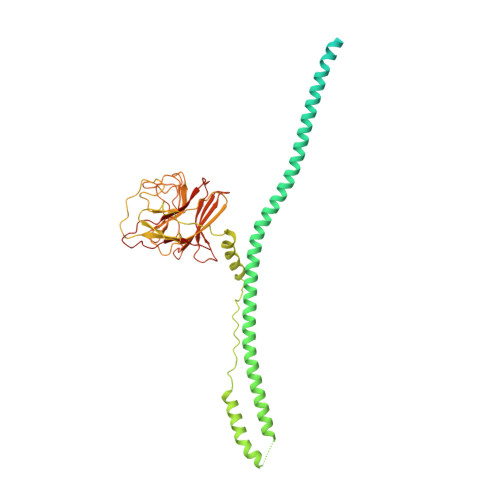
Cryo-EM structure of human MG53 homodimer.
Niu, Y., Chen, G., Lv, F., Xiao, R.P., Hu, X., Chen, L.(2022) Biochem J 479: 1909-1916
- PubMed: 36053137
- DOI: https://doi.org/10.1042/BCJ20220385
- Primary Citation of Related Structures:
7Y4S - PubMed Abstract:
MG53 is a tripartite motif (TRIM) family E3 ligase and plays important biological functions. Here we present the cryo-EM structure of human MG53, showing that MG53 is a homodimer consisting of a 'body' and two 'wings'. Intermolecular interactions are mainly distributed in the 'body' which is relatively stable, while two 'wings' are more dynamic. The overall architecture of MG53 is distinct from those of TRIM20 and TRIM25, illustrating the broad structural diversity of this protein family.
- State Key Laboratory of Membrane Biology, College of Future Technology, Institute of Molecular Medicine, Beijing Key Laboratory of Cardiometabolic Molecular Medicine, Peking University, Beijing 100871, China.
Organizational Affiliation: