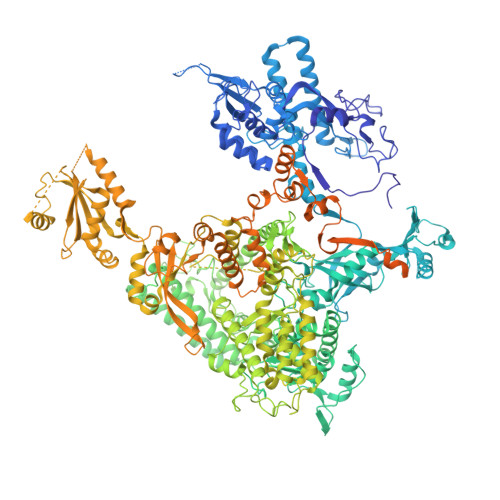
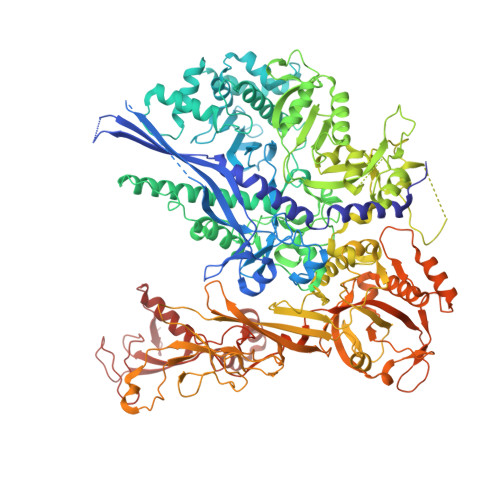
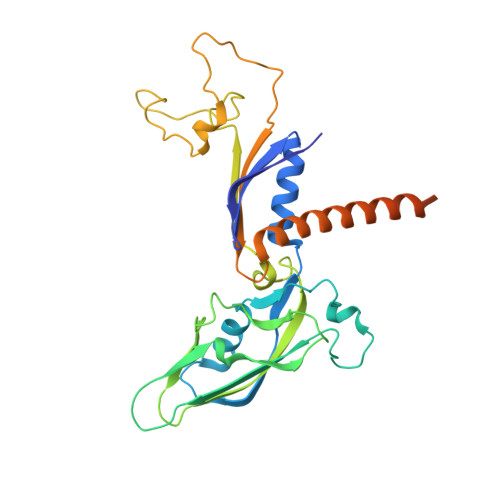
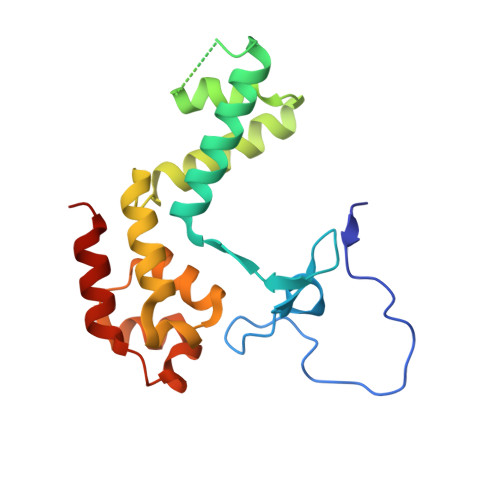
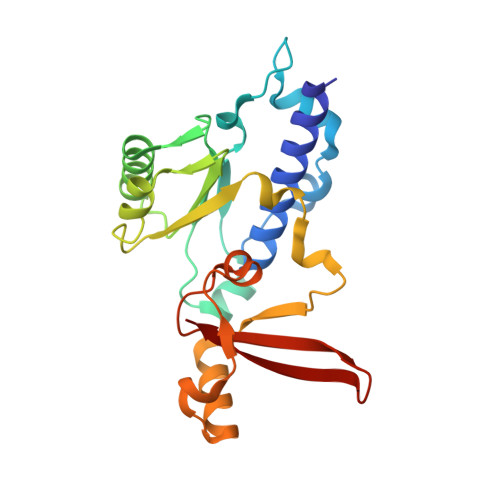
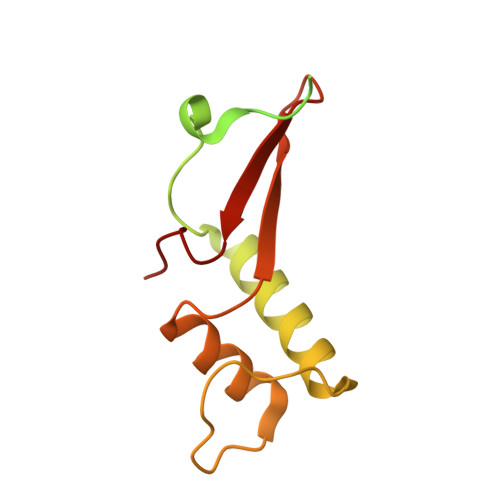
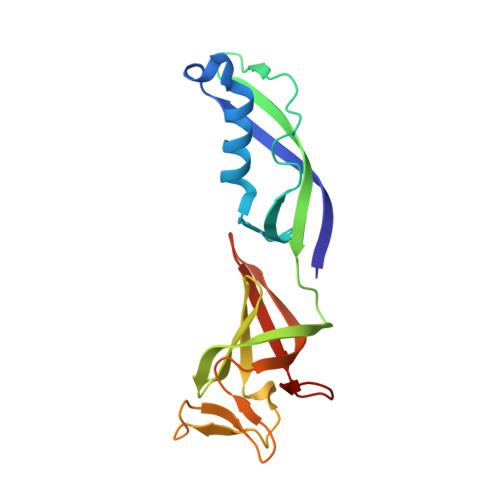
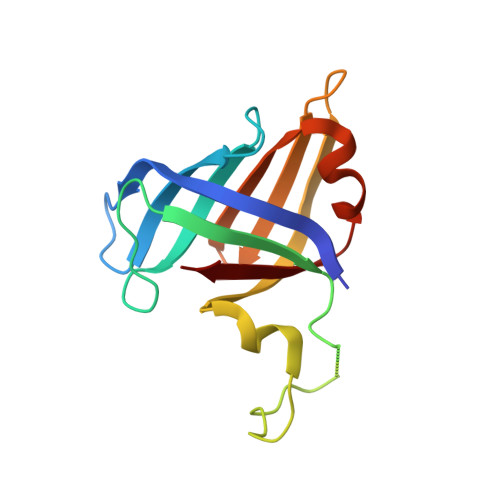
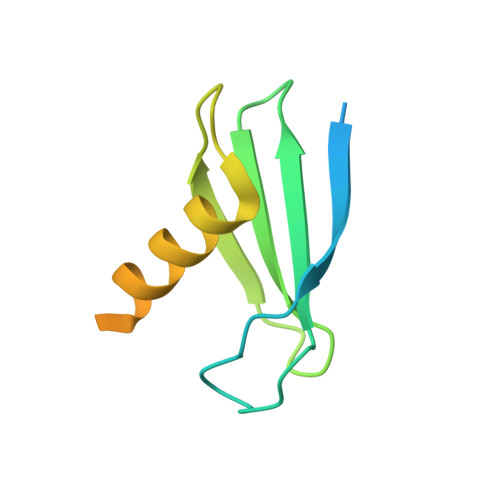
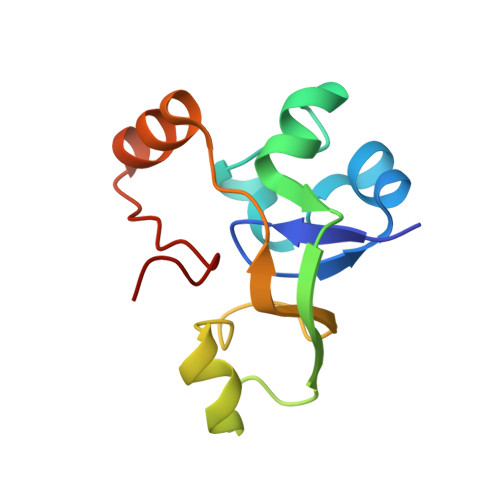
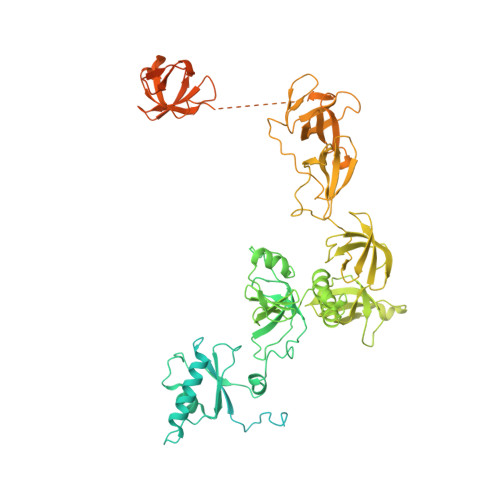
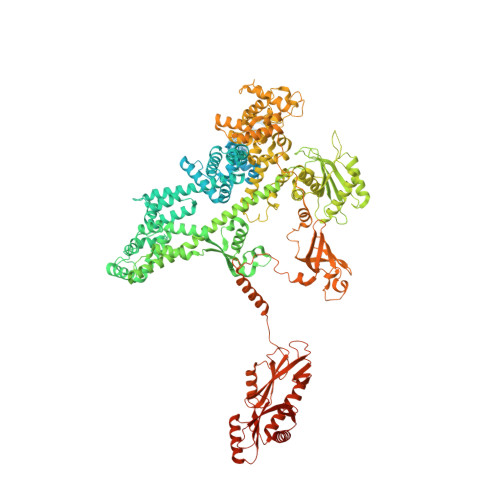
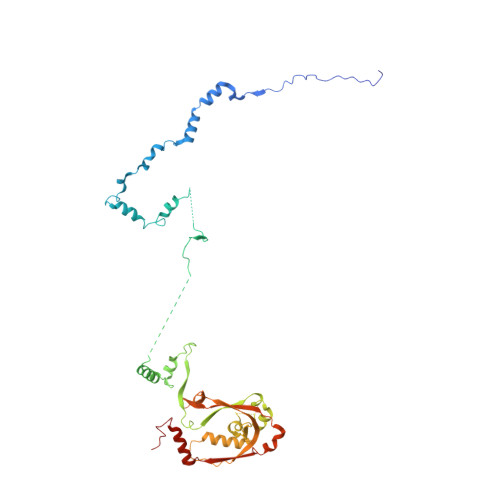
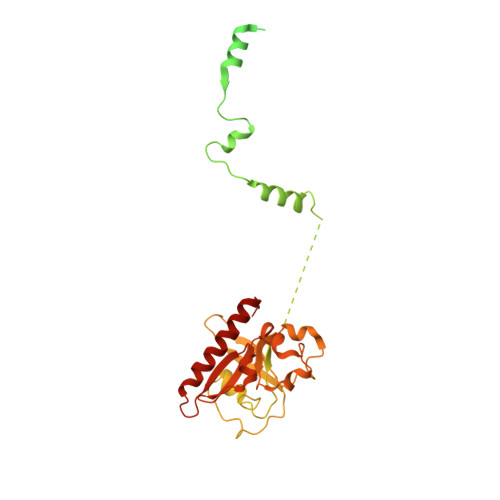
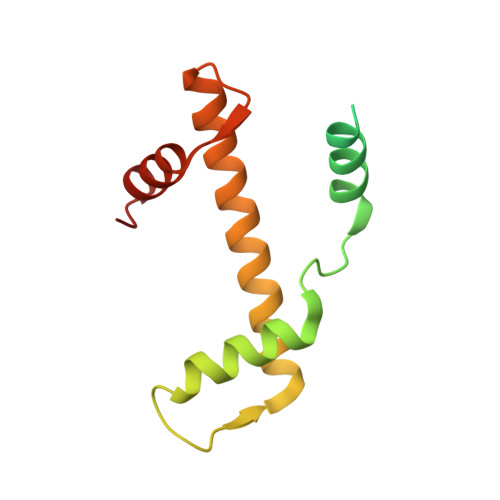
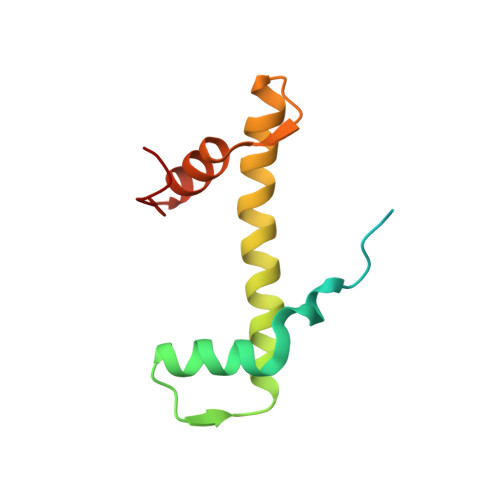
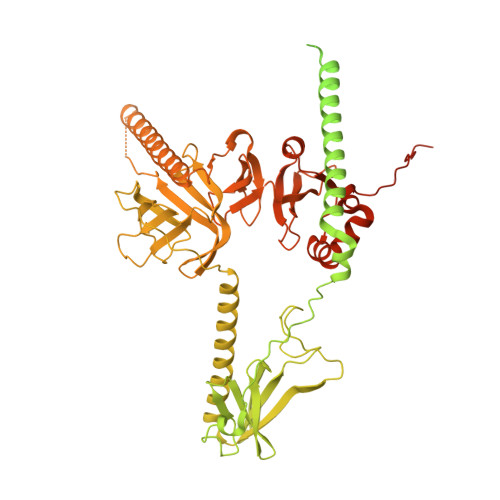
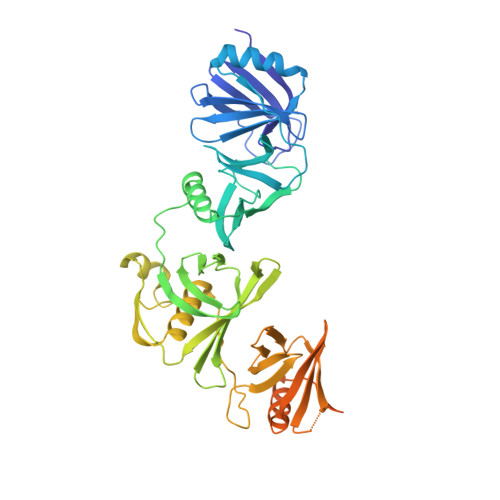
Structural basis of nucleosome disassembly and reassembly by RNAPII elongation complex with FACT.
Ehara, H., Kujirai, T., Shirouzu, M., Kurumizaka, H., Sekine, S.I.(2022) Science 377: eabp9466-eabp9466
- PubMed: 35981082
- DOI: https://doi.org/10.1126/science.abp9466
- Primary Citation of Related Structures:
7XN7, 7XSE, 7XSX, 7XSZ, 7XT7, 7XTD, 7XTI - PubMed Abstract:
During gene transcription, RNA polymerase II (RNAPII) traverses nucleosomes in chromatin, but the mechanism has remained elusive. Using cryo-electron microscopy, we obtained structures of the RNAPII elongation complex (EC) passing through a nucleosome in the presence of the transcription elongation factors Spt6, Spn1, Elf1, Spt4/5, and Paf1C and the histone chaperone FACT (facilitates chromatin transcription). The structures show snapshots of EC progression on DNA mediating downstream nucleosome disassembly, followed by its reassembly upstream of the EC, which is facilitated by FACT. FACT dynamically adapts to successively occurring subnucleosome intermediates, forming an interface with the EC. Spt6, Spt4/5, and Paf1C form a "cradle" at the EC DNA-exit site and support the upstream nucleosome reassembly. These structures explain the mechanism by which the EC traverses nucleosomes while maintaining the chromatin structure and epigenetic information.
- RIKEN Center for Biosystems Dynamics Research, Tsurumi-ku, Yokohama 230-0045, Japan.
Organizational Affiliation: