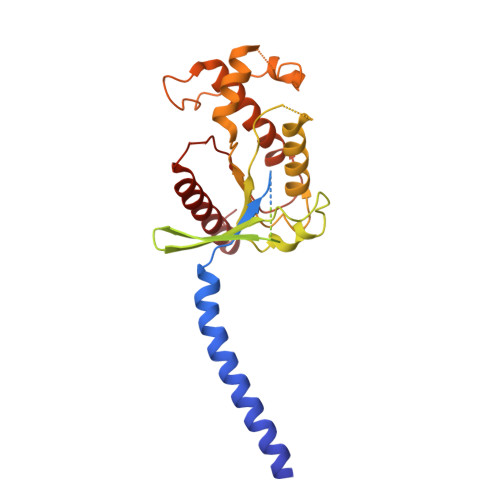
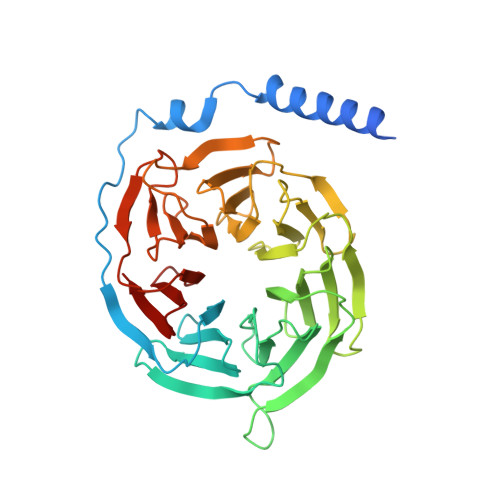
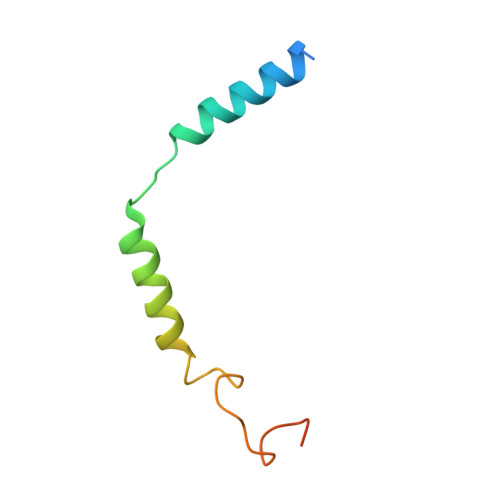
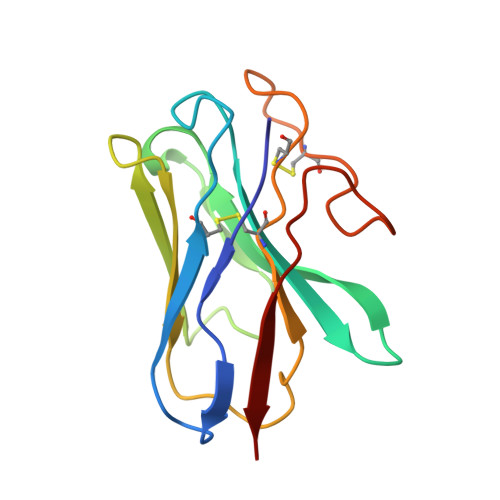
Tethered peptide activation mechanism of the adhesion GPCRs ADGRG2 and ADGRG4.
Xiao, P., Guo, S., Wen, X., He, Q.T., Lin, H., Huang, S.M., Gou, L., Zhang, C., Yang, Z., Zhong, Y.N., Yang, C.C., Li, Y., Gong, Z., Tao, X.N., Yang, Z.S., Lu, Y., Li, S.L., He, J.Y., Wang, C., Zhang, L., Kong, L., Sun, J.P., Yu, X.(2022) Nature 604: 771-778
- PubMed: 35418677
- DOI: https://doi.org/10.1038/s41586-022-04590-8
- Primary Citation of Related Structures:
7WUI, 7WUJ, 7WUQ - PubMed Abstract:
Adhesion G protein-coupled receptors (aGPCRs) constitute an evolutionarily ancient family of receptors that often undergo autoproteolysis to produce α and β subunits 1-3 . A tethered agonism mediated by the 'Stachel sequence' of the β subunit has been proposed to have central roles in aGPCR activation 4-6 . Here we present three cryo-electron microscopy structures of aGPCRs coupled to the G s heterotrimer. Two of these aGPCRs are activated by tethered Stachel sequences-the ADGRG2-β-G s complex and the ADGRG4-β-G s complex (in which β indicates the β subunit of the aGPCR)-and the other is the full-length ADGRG2 in complex with the exogenous ADGRG2 Stachel-sequence-derived peptide agonist IP15 (ADGRG2(FL)-IP15-G s ). The Stachel sequences of both ADGRG2-β and ADGRG4-β assume a U shape and insert deeply into the seven-transmembrane bundles. Constituting the FXφφφXφ motif (in which φ represents a hydrophobic residue), five residues of ADGRG2-β or ADGRG4-β extend like fingers to mediate binding to the seven-transmembrane domain and activation of the receptor. The structure of the ADGRG2(FL)-IP15-G s complex reveals the structural basis for the improved binding affinity of IP15 compared with VPM-p15 and indicates that rational design of peptidic agonists could be achieved by exploiting aGPCR-β structures. By converting the 'finger residues' to acidic residues, we develop a method to generate peptidic antagonists towards several aGPCRs. Collectively, our study provides structural and biochemical insights into the tethered activation mechanism of aGPCRs.
- Department of Clinical Laboratory, The Second Hospital, and Advanced Medical Research Institute, Cheeloo College of Medicine, Shandong University, Jinan, China.
Organizational Affiliation: