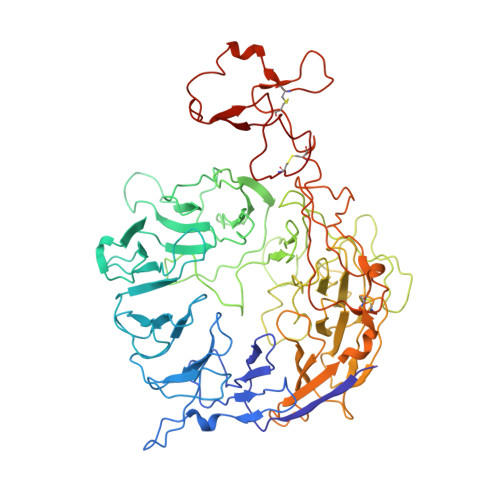
Cryo-EM structures reveal distinct apo conformations of sortilin-related receptor SORLA.
Zhang, X., Wu, C., Song, Z., Sun, D., Zhai, L., Liu, C.(2022) Biochem Biophys Res Commun 600: 75-79
- PubMed: 35196630
- DOI: https://doi.org/10.1016/j.bbrc.2022.01.108
- Primary Citation of Related Structures:
7VT0 - PubMed Abstract:
Sorting-related receptor with A-type repeats (SORLA) is an important receptor for regulating normal cellular functions via protein sorting. Here, we determined the structures of the full-length SORLA and identified two distinct conformations of apo-SORLA using single-particle cryogenic electron microscopy. In contrast to homologous proteins, both monomer and dimer forms of SORLA existed in a neutral solution. Only three hydrogen bonds in the vicinity of the dimer interface implied the involvement in dimerization. The orientation of residue R490 was a key point for ligand binding. These results suggest a unique mechanism of SORLA dimerization for protein trafficking.
- Department of Gastroenterology, The First Affiliated Hospital of Shenzhen University, Shenzhen People's Second Hospital, Shenzhen, 518000, China; Institute of Synthetic Biology, Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen, 518055, China; Cryo-EM Centre, Southern University of Science and Technology, Shenzhen, 518055, China. Electronic address: 11649008@mail.sustech.edu.cn.
Organizational Affiliation: