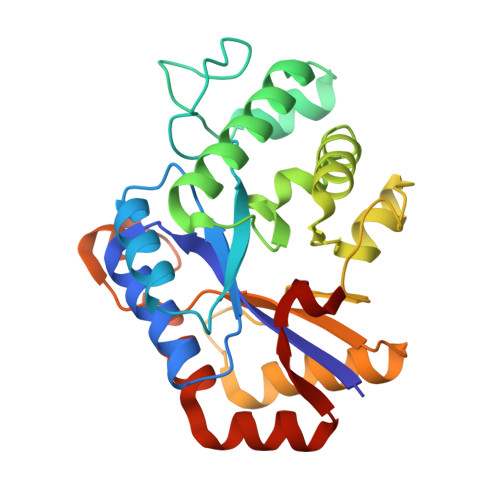
Crystal structure of ChbG from Klebsiella pneumoniae reveals the molecular basis of diacetylchitobiose deacetylation.
Lee, S.Y., Pardhe, B.D., Oh, T.J., Park, H.H.(2022) Commun Biol 5: 862-862
- PubMed: 36002585
- DOI: https://doi.org/10.1038/s42003-022-03824-9
- Primary Citation of Related Structures:
7VI8 - PubMed Abstract:
The chitobiose (chb) operon is involved in the synthesis of chitooligosaccharide and is comprised of a BCARFG gene cluster. ChbG encodes a chitooligosaccharide deacetylase (CDA) which catalyzes the removal of one acetyl group from N,N'-diacetylchitobiose. It is considered a novel type of CDA due to its lack of sequence homology. Although there are various structural studies of CDAs linked to the kinetic properties of the enzyme, the structural information of ChbG is unavailable. In this study, the crystal structure of ChbG from Klebsiella pneumoniae is provided. The molecular basis of deacetylation of diacetylchitobiose by ChbG is determined based on structural analysis, mutagenesis, biophysical analysis, and in silico docking of the substrate, diacetylchitobiose. This study contributes towards a deeper understanding of chitin and chitosan biology, as well as provides a platform to engineer CDA biocatalysts.
- College of Pharmacy, Chung-Ang University, Seoul, 06974, Republic of Korea.
Organizational Affiliation: