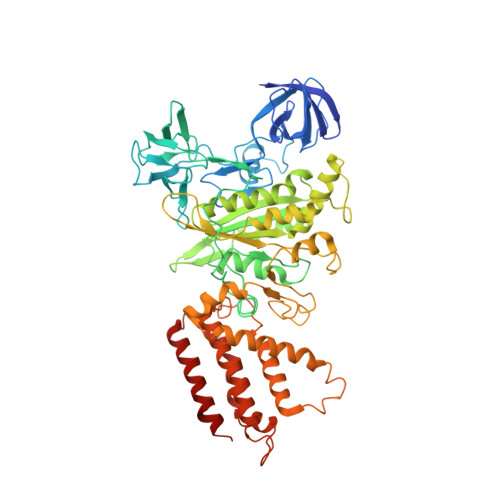
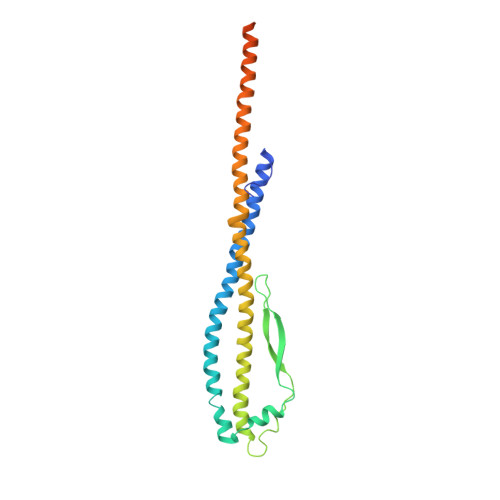
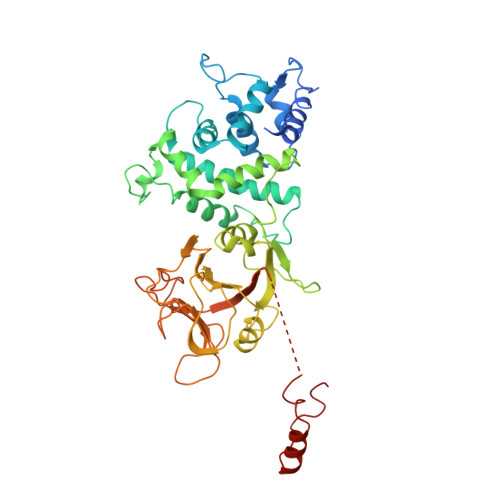
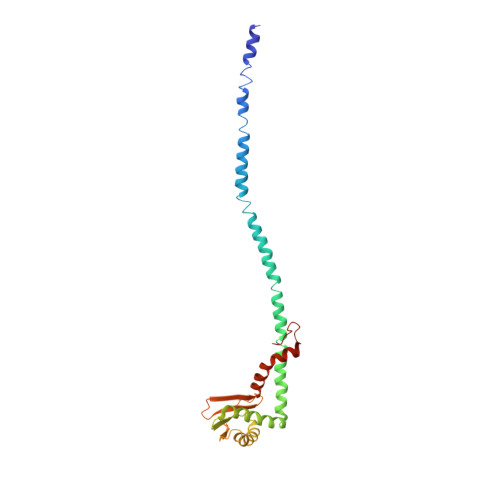
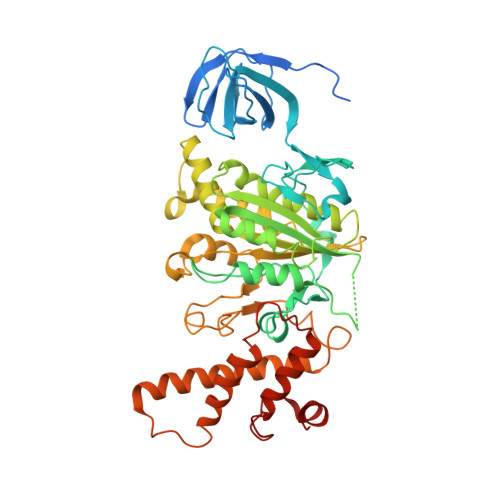
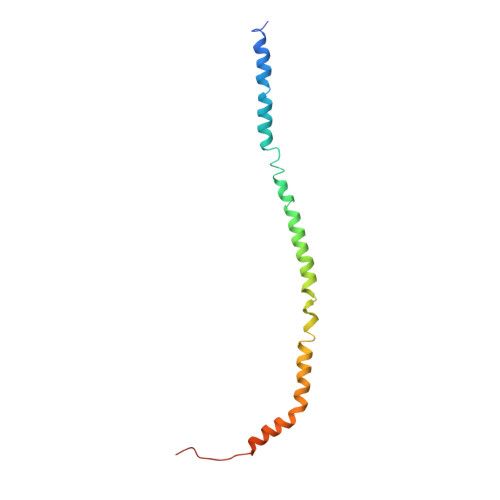
Molecular basis of mEAK7-mediated human V-ATPase regulation.
Wang, R., Qin, Y., Xie, X.S., Li, X.(2022) Nat Commun 13: 3272-3272
- PubMed: 35672408
- DOI: https://doi.org/10.1038/s41467-022-30899-z
- Primary Citation of Related Structures:
7UNE, 7UNF - PubMed Abstract:
The activity of V-ATPase is well-known to be regulated by reversible dissociation of its V 1 and V o domains in response to growth factor stimulation, nutrient sensing, and cellular differentiation. The molecular basis of its regulation by an endogenous modulator without affecting V-ATPase assembly remains unclear. Here, we discover that a lysosome-anchored protein termed (mammalian Enhancer-of-Akt-1-7 (mEAK7)) binds to intact V-ATPase. We determine cryo-EM structure of human mEAK7 in complex with human V-ATPase in native lipid-containing nanodiscs. The structure reveals that the TLDc domain of mEAK7 engages with subunits A, B, and E, while its C-terminal domain binds to subunit D, presumably blocking V 1 -V o torque transmission. Our functional studies suggest that mEAK7, which may act as a V-ATPase inhibitor, does not affect the activity of V-ATPase in vitro. However, overexpression of mEAK7 in HCT116 cells that stably express subunit a4 of V-ATPase represses the phosphorylation of ribosomal protein S6. Thus, this finding suggests that mEAK7 potentially links mTOR signaling with V-ATPase activity.
- Department of Molecular Genetics, University of Texas Southwestern Medical Center, Dallas, TX, 75390, USA.
Organizational Affiliation: