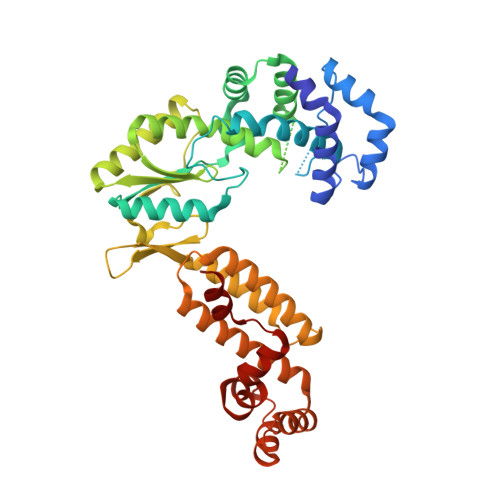
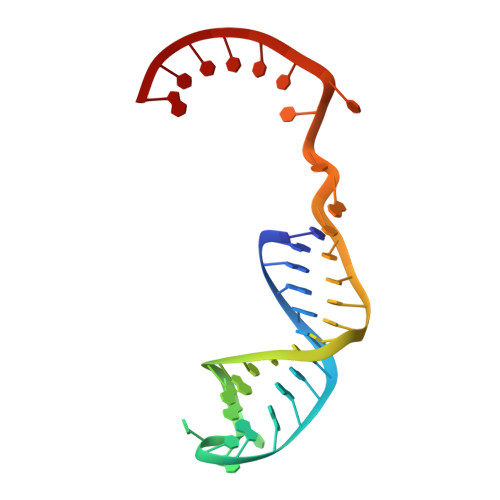
Structures of a mobile intron retroelement poised to attack its structured DNA target
Chung, K., Xu, L., Chai, P., Peng, J., Devarkar, S.C., Pyle, A.M.(2022) Science 378: 627-634
- PubMed: 36356138
- DOI: https://doi.org/10.1126/science.abq2844
- Primary Citation of Related Structures:
7UIM, 7UIN - PubMed Abstract:
Group II introns are ribozymes that catalyze their self-excision and function as retroelements that invade DNA. As retrotransposons, group II introns form ribonucleoprotein (RNP) complexes that roam the genome, integrating by reversal of forward splicing. Here we show that retrotransposition is achieved by a tertiary complex between a structurally elaborate ribozyme, its protein mobility factor, and a structured DNA substrate. We solved cryo-electron microscopy structures of an intact group IIC intron-maturase retroelement that was poised for integration into a DNA stem-loop motif. By visualizing the RNP before and after DNA targeting, we show that it is primed for attack and fits perfectly with its DNA target. This study reveals design principles of a prototypical retroelement and reinforces the hypothesis that group II introns are ancient elements of genetic diversification.
- Department of Molecular Biophysics and Biochemistry, Yale University, New Haven, CT 06511, USA.
Organizational Affiliation: