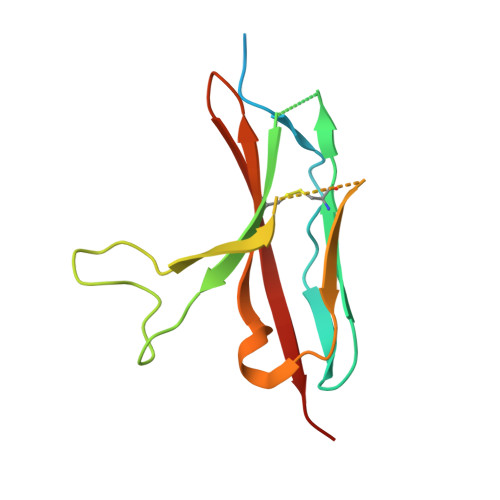
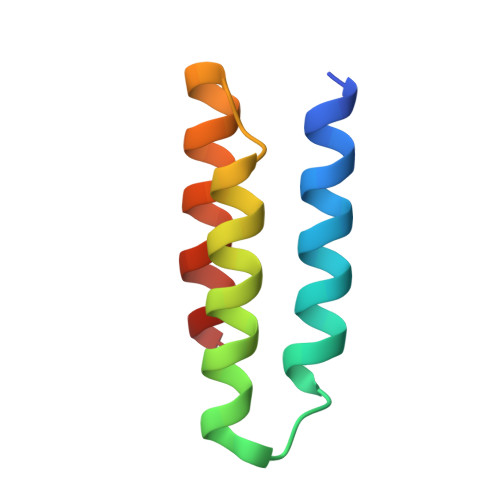
Isoform-specific inhibition of FGFR signaling achieved by a de-novo-designed mini-protein.
Park, J.S., Choi, J., Cao, L., Mohanty, J., Suzuki, Y., Park, A., Baker, D., Schlessinger, J., Lee, S.(2022) Cell Rep 41: 111545-111545
- PubMed: 36288716
- DOI: https://doi.org/10.1016/j.celrep.2022.111545
- Primary Citation of Related Structures:
7TYD - PubMed Abstract:
Cellular signaling by fibroblast growth factor receptors (FGFRs) is a highly regulated process mediated by specific interactions between distinct subsets of fibroblast growth factor (FGF) ligands and two FGFR isoforms generated by alternative splicing: an epithelial b- and mesenchymal c-isoforms. Here, we investigate the properties of a mini-protein, mb7, developed by an in silico design strategy to bind to the ligand-binding region of FGFR2. We describe structural, biophysical, and cellular analyses demonstrating that mb7 binds with high affinity to the c-isoforms of FGFR, resulting in inhibition of cellular signaling induced by a subset of FGFs that preferentially activate c-isoforms of FGFR. Notably, as mb7 blocks interaction between FGFR with Klotho proteins, it functions as an antagonist of the metabolic hormones FGF19 and FGF21, providing mechanistic insights and strategies for the development of therapeutics for diseases driven by aberrantly activated FGFRs.
- Department of Pharmacology, Yale University School of Medicine, New Haven, CT 06520, USA.
Organizational Affiliation: