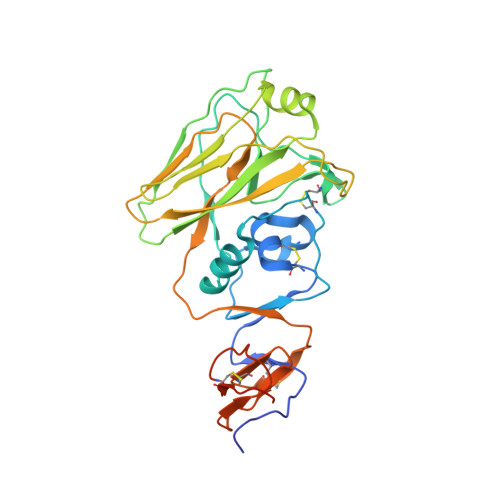
A new class of antibodies that overcomes a steric barrier to cross-group neutralization of influenza viruses.
Simmons, H.C., Watanabe, A., Oguin Iii, T.H., Van Itallie, E.S., Wiehe, K.J., Sempowski, G.D., Kuraoka, M., Kelsoe, G., McCarthy, K.R.(2023) PLoS Biol 21: e3002415-e3002415
- PubMed: 38127922
- DOI: https://doi.org/10.1371/journal.pbio.3002415
- Primary Citation of Related Structures:
7TRH, 7TRI - PubMed Abstract:
Antibody titers that inhibit the influenza virus hemagglutinin (HA) from engaging its receptor are the accepted correlate of protection from infection. Many potent antibodies with broad, intra-subtype specificity bind HA at the receptor binding site (RBS). One barrier to broad H1-H3 cross-subtype neutralization is an insertion (133a) between positions 133 and 134 on the rim of the H1 HA RBS. We describe here a class of antibodies that overcomes this barrier. These genetically unrestricted antibodies are abundant in the human B cell memory compartment. Analysis of the affinities of selected members of this class for historical H1 and H3 isolates suggest that they were elicited by H3 exposure and broadened or diverted by later exposure(s) to H1 HA. RBS mutations in egg-adapted vaccine strains cause the new H1 specificity of these antibodies to depend on the egg adaptation. The results suggest that suitable immunogens might elicit 133a-independent, H1-H3 cross neutralization by RBS-directed antibodies.
- Center for Vaccine Research, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, United States of America.
Organizational Affiliation: