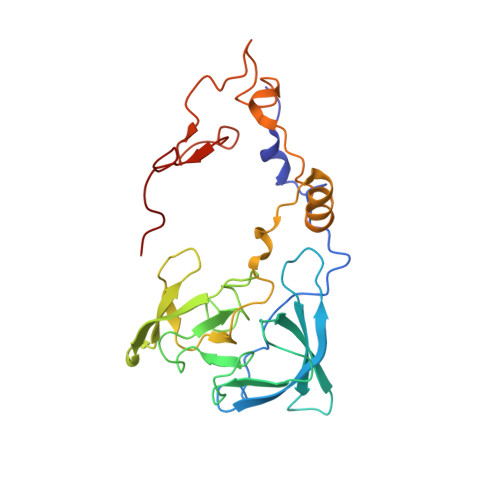
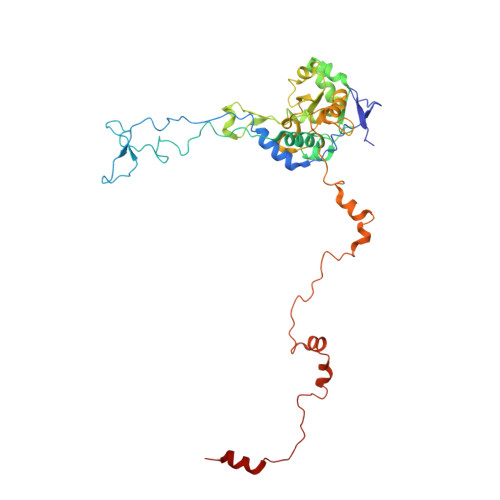
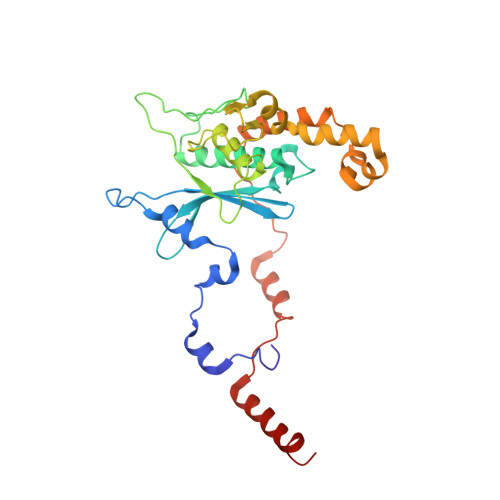
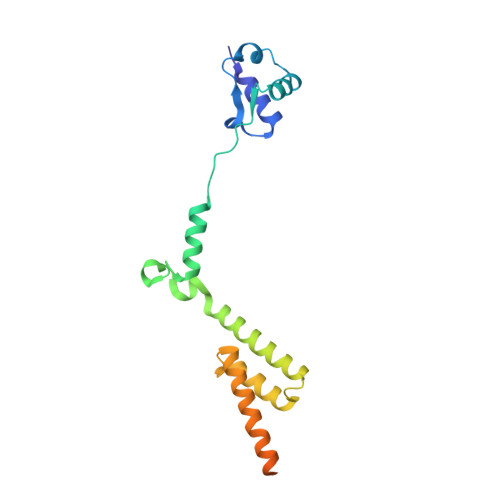
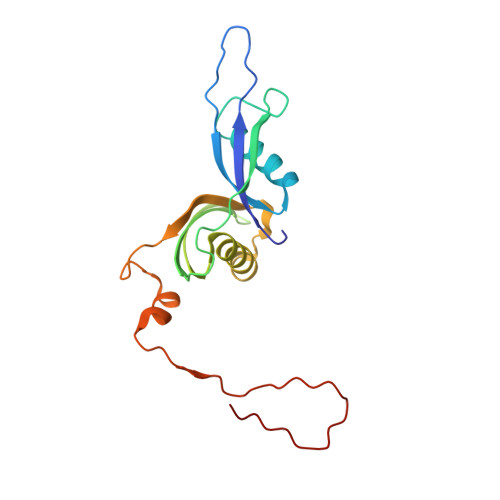
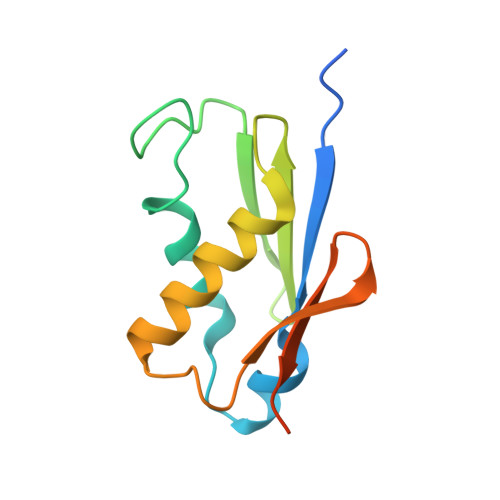
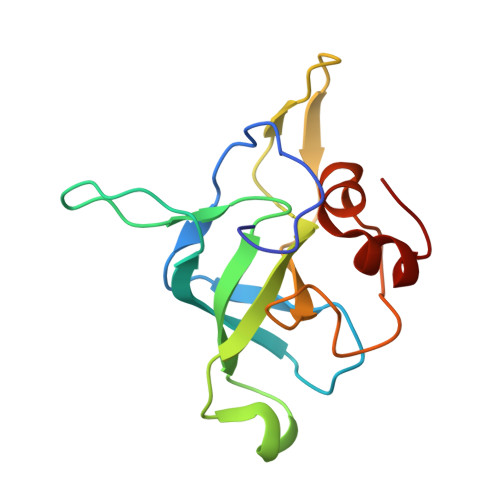
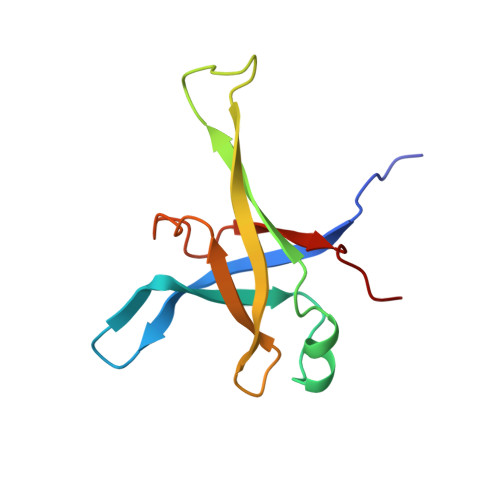
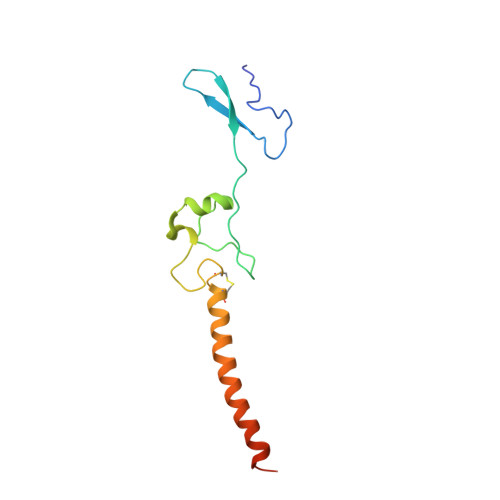
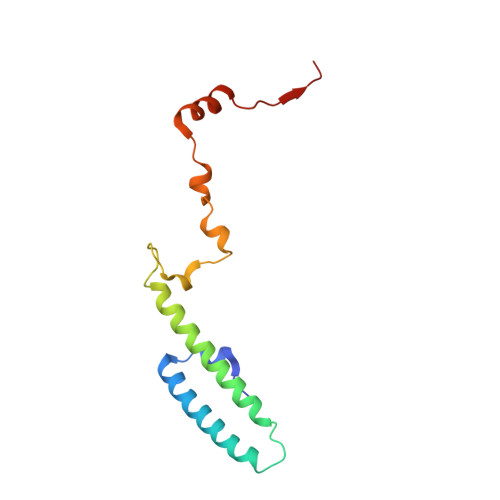
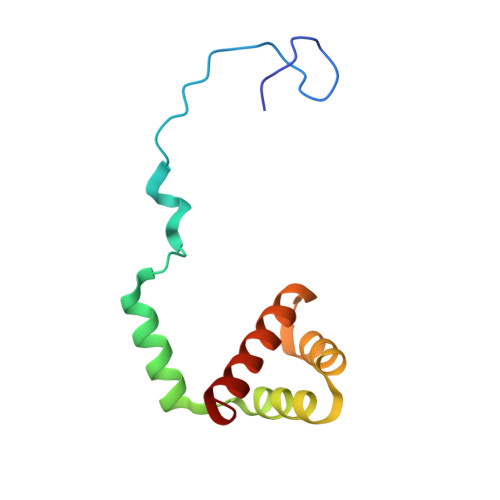
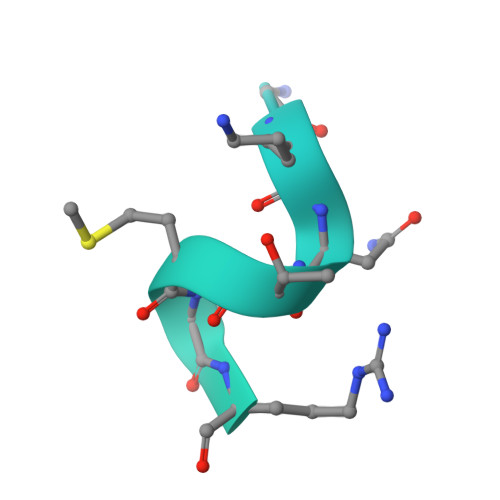
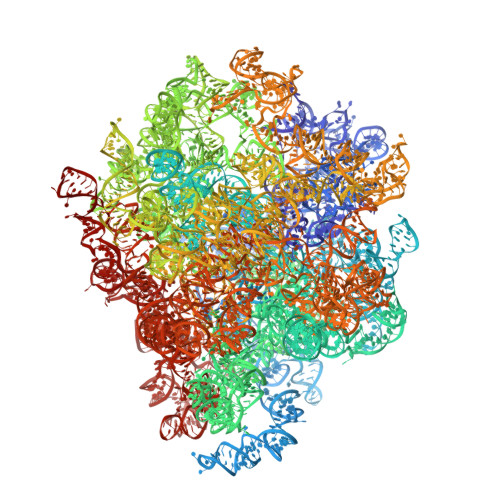
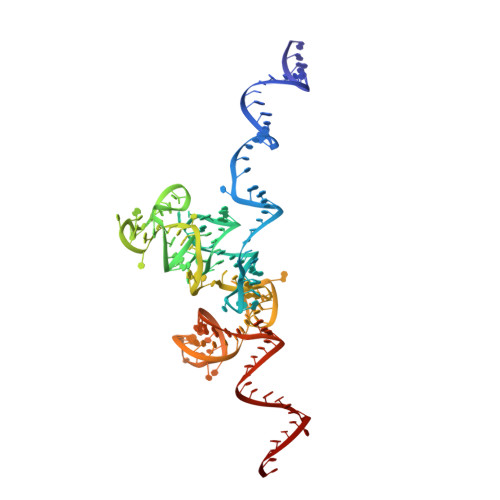
Ribosome inhibition by C9ORF72-ALS/FTD-associated poly-PR and poly-GR proteins revealed by cryo-EM.
Loveland, A.B., Svidritskiy, E., Susorov, D., Lee, S., Park, A., Zvornicanin, S., Demo, G., Gao, F.B., Korostelev, A.A.(2022) Nat Commun 13: 2776-2776
- PubMed: 35589706
- DOI: https://doi.org/10.1038/s41467-022-30418-0
- Primary Citation of Related Structures:
7TOO, 7TOP, 7TOQ, 7TOR, 7TOS - PubMed Abstract:
Toxic dipeptide-repeat (DPR) proteins are produced from expanded G 4 C 2 repeats in the C9ORF72 gene, the most common genetic cause of amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD). Two DPR proteins, poly-PR and poly-GR, repress cellular translation but the molecular mechanism remains unknown. Here we show that poly-PR and poly-GR of ≥20 repeats inhibit the ribosome's peptidyl-transferase activity at nanomolar concentrations, comparable to specific translation inhibitors. High-resolution cryogenic electron microscopy (cryo-EM) reveals that poly-PR and poly-GR block the polypeptide tunnel of the ribosome, extending into the peptidyl-transferase center (PTC). Consistent with these findings, the macrolide erythromycin, which binds in the tunnel, competes with poly-PR and restores peptidyl-transferase activity. Our results demonstrate that strong and specific binding of poly-PR and poly-GR in the ribosomal tunnel blocks translation, revealing the structural basis of their toxicity in C9ORF72-ALS/FTD.
- RNA Therapeutics Institute, UMass Chan Medical School, 368 Plantation Street, Worcester, MA, 01605, USA.
Organizational Affiliation: