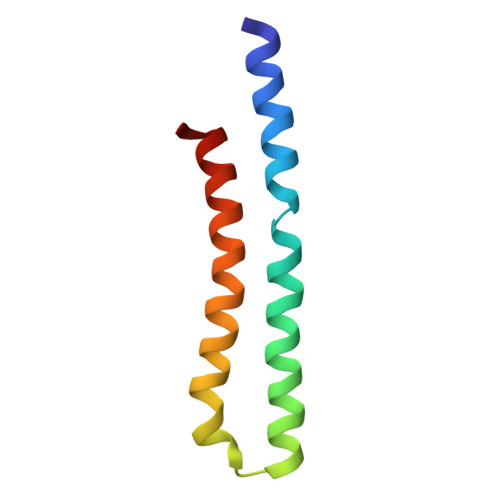
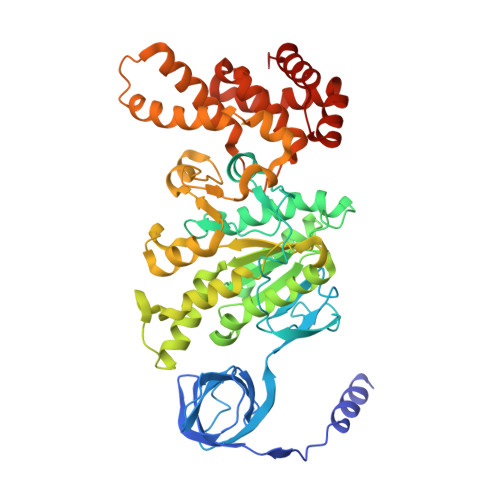
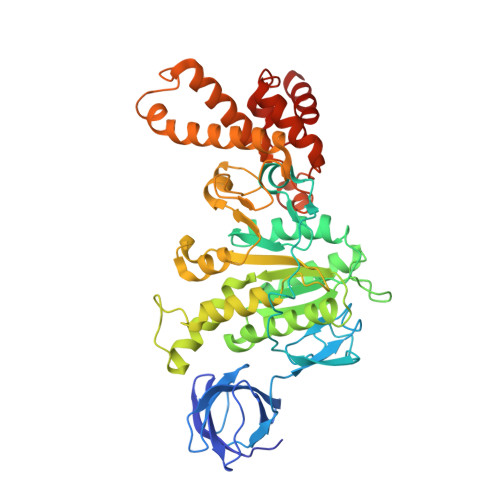
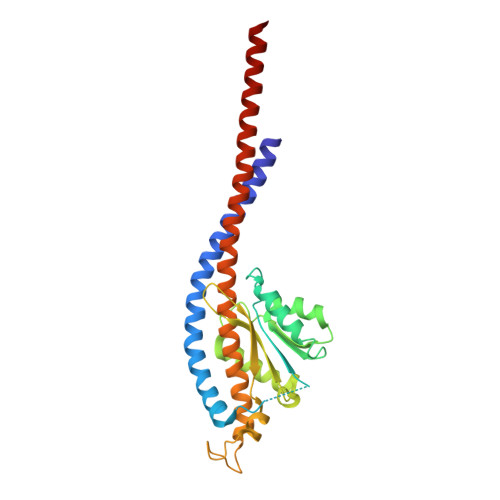
Structure of ATP synthase under strain during catalysis.
Guo, H., Rubinstein, J.L.(2022) Nat Commun 13: 2232-2232
- PubMed: 35468906
- DOI: https://doi.org/10.1038/s41467-022-29893-2
- Primary Citation of Related Structures:
7TJS, 7TJT, 7TJU, 7TJV, 7TJW, 7TJX, 7TJY, 7TJZ, 7TK0, 7TK1, 7TK2, 7TK3, 7TK4, 7TK5, 7TK6, 7TK7, 7TK8, 7TK9, 7TKA, 7TKB, 7TKC, 7TKD, 7TKE, 7TKF, 7TKG, 7TKH, 7TKI, 7TKJ, 7TKK, 7TKL, 7TKM, 7TKN, 7TKO, 7TKP, 7TKQ, 7TKR, 7TKS - PubMed Abstract:
ATP synthases are macromolecular machines consisting of an ATP-hydrolysis-driven F 1 motor and a proton-translocation-driven F O motor. The F 1 and F O motors oppose each other's action on a shared rotor subcomplex and are held stationary relative to each other by a peripheral stalk. Structures of resting mitochondrial ATP synthases revealed a left-handed curvature of the peripheral stalk even though rotation of the rotor, driven by either ATP hydrolysis in F 1 or proton translocation through F O , would apply a right-handed bending force to the stalk. We used cryoEM to image yeast mitochondrial ATP synthase under strain during ATP-hydrolysis-driven rotary catalysis, revealing a large deformation of the peripheral stalk. The structures show how the peripheral stalk opposes the bending force and suggests that during ATP synthesis proton translocation causes accumulation of strain in the stalk, which relaxes by driving the relative rotation of the rotor through six sub-steps within F 1 , leading to catalysis.
- Molecular Medicine Program, The Hospital for Sick Children, Toronto, Ontario, Canada.
Organizational Affiliation: