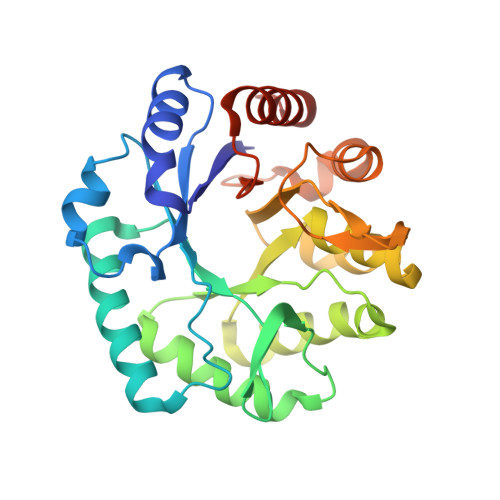
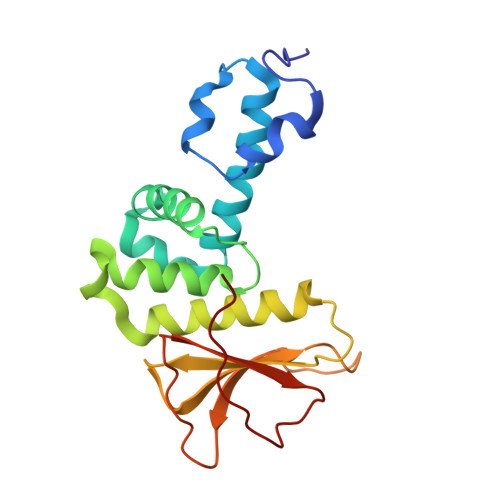
A mixed-valent Fe(II)Fe(III) species converts cysteine to an oxazolone/thioamide pair in methanobactin biosynthesis.
Park, Y.J., Jodts, R.J., Slater, J.W., Reyes, R.M., Winton, V.J., Montaser, R.A., Thomas, P.M., Dowdle, W.B., Ruiz, A., Kelleher, N.L., Bollinger Jr., J.M., Krebs, C., Hoffman, B.M., Rosenzweig, A.C.(2022) Proc Natl Acad Sci U S A 119: e2123566119-e2123566119
- PubMed: 35320042
- DOI: https://doi.org/10.1073/pnas.2123566119
- Primary Citation of Related Structures:
7TCR, 7TCU, 7TCW, 7TCX - PubMed Abstract:
SignificanceMethanobactins (Mbns), copper-binding peptidic compounds produced by some bacteria, are candidate therapeutics for human diseases of copper overload. The paired oxazolone-thioamide bidentate ligands of methanobactins are generated from cysteine residues in a precursor peptide, MbnA, by the MbnBC enzyme complex. MbnBC activity depends on the presence of iron and oxygen, but the catalytically active form has not been identified. Here, we provide evidence that a dinuclear Fe(II)Fe(III) center in MbnB, which is the only representative of a >13,000-member protein family to be characterized, is responsible for this reaction. These findings expand the known roles of diiron enzymes in biology and set the stage for mechanistic understanding, and ultimately engineering, of the MbnBC biosynthetic complex.
- Department of Molecular Biosciences, Northwestern University, Evanston, IL 60208.
Organizational Affiliation: