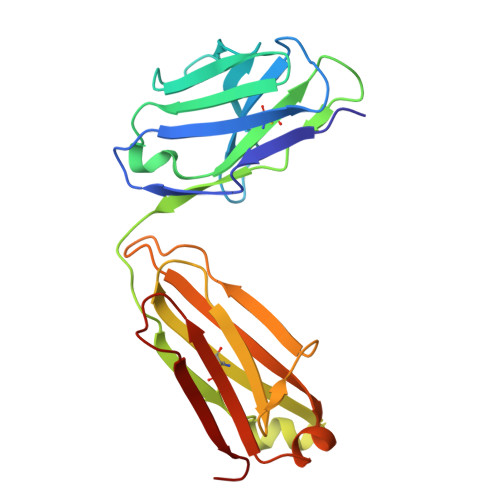
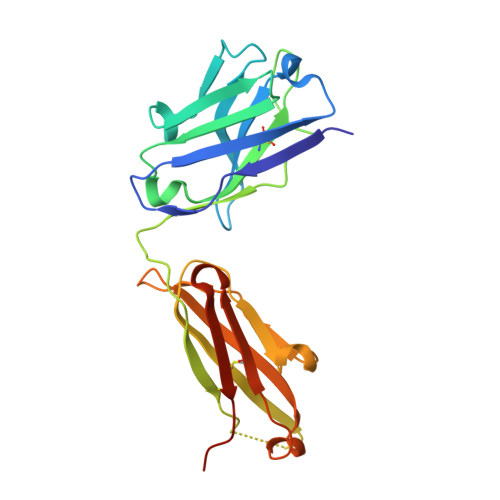
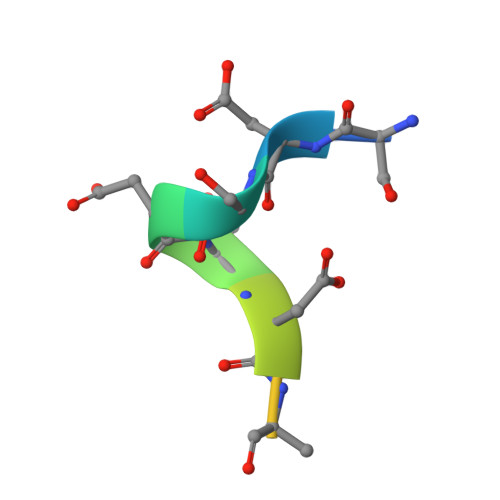
Multivalent human antibody-centyrin fusion protein to prevent and treat Staphylococcus aureus infections.
Buckley, P.T., Chan, R., Fernandez, J., Luo, J., Lacey, K.A., DuMont, A.L., O'Malley, A., Brezski, R.J., Zheng, S., Malia, T., Whitaker, B., Zwolak, A., Payne, A., Clark, D., Sigg, M., Lacy, E.R., Kornilova, A., Kwok, D., McCarthy, S., Wu, B., Morrow, B., Nemeth-Seay, J., Petley, T., Wu, S., Strohl, W.R., Lynch, A.S., Torres, V.J.(2023) Cell Host Microbe 31: 751-765.e11
- PubMed: 37098341
- DOI: https://doi.org/10.1016/j.chom.2023.04.004
- Primary Citation of Related Structures:
7T82, 7T86, 7T87 - PubMed Abstract:
Treating and preventing infections by antimicrobial-resistant bacterial pathogens is a worldwide problem. Pathogens such as Staphylococcus aureus produce an array of virulence determinants, making it difficult to identify single targets for the development of vaccines or monoclonal therapies. We described a human-derived anti-S. aureus monoclonal antibody (mAb)-centyrin fusion protein ("mAbtyrin") that simultaneously targets multiple bacterial adhesins, resists proteolysis by bacterial protease GluV8, avoids Fc engagement by S. aureus IgG-binding proteins SpA and Sbi, and neutralizes pore-forming leukocidins via fusion with anti-toxin centyrins, while maintaining Fc- and complement-mediated functions. Compared with the parental mAb, mAbtyrin protected human phagocytes and boosted phagocyte-mediated killing. The mAbtyrin also reduced pathology, reduced bacterial burden, and protected from different types of infections in preclinical animal models. Finally, mAbtyrin synergized with vancomycin, enhancing pathogen clearance in an animal model of bacteremia. Altogether, these data establish the potential of multivalent mAbs for treating and preventing S. aureus diseases.
- Janssen Research & Development, 1400 McKean Road, Spring House, PA, USA. Electronic address: pbuckle5@its.jnj.com.
Organizational Affiliation: