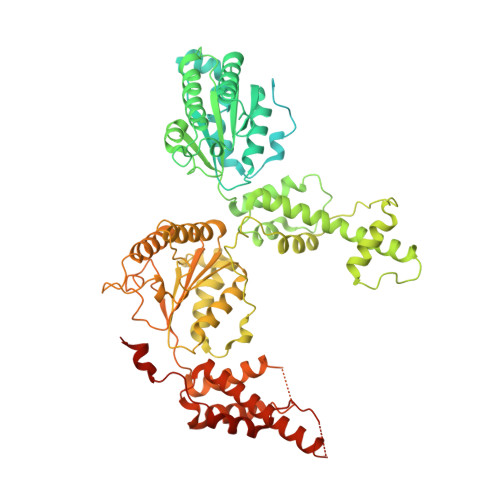
Communication network within the essential AAA-ATPase Rix7 drives ribosome assembly.
Kocaman, S., Lo, Y.H., Krahn, J.M., Sobhany, M., Dandey, V.P., Petrovich, M.L., Etigunta, S.K., Williams, J.G., Deterding, L.J., Borgnia, M.J., Stanley, R.E.(2022) PNAS Nexus 1: pgac118-pgac118
- PubMed: 36090660
- DOI: https://doi.org/10.1093/pnasnexus/pgac118
- Primary Citation of Related Structures:
7SWL, 7T0V, 7T3I - PubMed Abstract:
Rix7 is an essential AAA+ ATPase that functions during the early stages of ribosome biogenesis. Rix7 is composed of three domains including an N-terminal domain (NTD) and two AAA+ domains (D1 and D2) that assemble into an asymmetric stacked hexamer. It was recently established that Rix7 is a presumed protein translocase that removes substrates from preribosomes by translocating them through its central pore. However, how the different domains of Rix7 coordinate their activities within the overall hexameric structure was unknown. We captured cryo-electron microscopy (EM) structures of single and double Walker B variants of full length Rix7. The disordered NTD was not visible in the cryo-EM reconstructions, but cross-linking mass spectrometry revealed that the NTD can associate with the central channel in vitro. Deletion of the disordered NTD enabled us to obtain a structure of the Rix7 hexamer to 2.9 Å resolution, providing high resolution details of critical motifs involved in substrate translocation and interdomain communication. This structure coupled with cell-based assays established that the linker connecting the D1 and D2 domains as well as the pore loops lining the central channel are essential for formation of the large ribosomal subunit. Together, our work shows that Rix7 utilizes a complex communication network to drive ribosome biogenesis.
- Department of Health and Human Services, Signal Transduction Laboratory, National Institute of Environmental Health Sciences, National Institutes of Health, 111 T. W. Alexander Drive, Research Triangle Park, NC 27709, USA.
Organizational Affiliation: