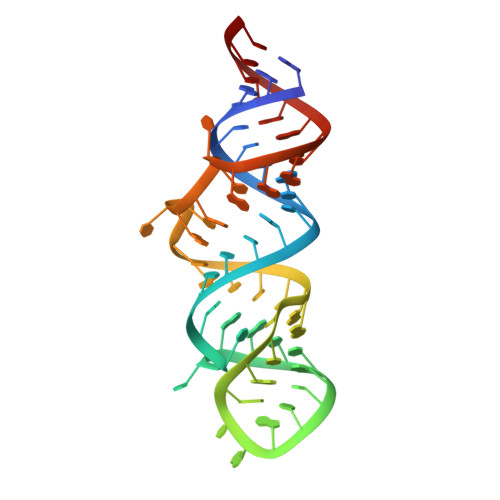
A structure-based mechanism for displacement of the HEXIM adapter from 7SK small nuclear RNA.
Pham, V.V., Gao, M., Meagher, J.L., Smith, J.L., D'Souza, V.M.(2022) Commun Biol 5: 819-819
- PubMed: 35970937
- DOI: https://doi.org/10.1038/s42003-022-03734-w
- Primary Citation of Related Structures:
7T1N, 7T1O, 7T1P - PubMed Abstract:
Productive transcriptional elongation of many cellular and viral mRNAs requires transcriptional factors to extract pTEFb from the 7SK snRNP by modulating the association between HEXIM and 7SK snRNA. In HIV-1, Tat binds to 7SK by displacing HEXIM. However, without the structure of the 7SK-HEXIM complex, the constraints that must be overcome for displacement remain unknown. Furthermore, while structure details of the Tat NL4-3 -7SK complex have been elucidated, it is unclear how subtypes with more HEXIM-like Tat sequences accomplish displacement. Here we report the structures of HEXIM, Tat G , and Tat Fin arginine rich motifs in complex with the apical stemloop-1 of 7SK. While most interactions between 7SK with HEXIM and Tat are similar, critical differences exist that guide function. First, the conformational plasticity of 7SK enables the formation of three different base pair configurations at a critical remodeling site, which allows for the modulation required for HEXIM binding and its subsequent displacement by Tat. Furthermore, the specific sequence variations observed in various Tat subtypes all converge on remodeling 7SK at this region. Second, we show that HEXIM primes its own displacement by causing specific local destabilization upon binding - a feature that is then exploited by Tat to bind 7SK more efficiently.
- Department of Molecular and Cellular Biology, Harvard University, Cambridge, MA, 02138, USA.
Organizational Affiliation: