Structure of Nicotinamide N-Methyltransferase (NNMT) in complex with inhibitor II329
Iyamu, I., Vilseck, J.Z., Yadav, R., Noinaj, N., Huang, R.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| NNMT protein | A [auth C], B [auth A], C [auth B], D | 283 | Homo sapiens | Mutation(s): 3 Gene Names: NNMT, hCG_39357 EC: 2.1.1.1 | 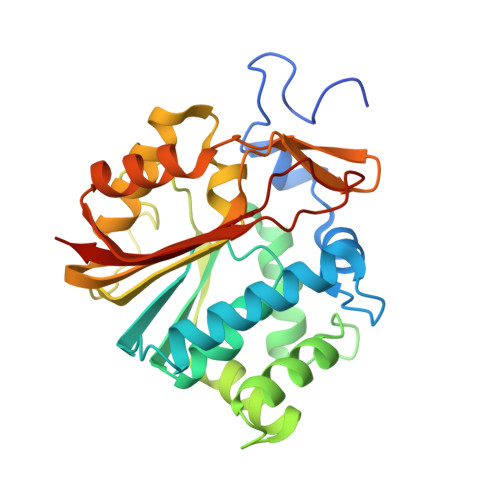 |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P40261 (Homo sapiens) Explore P40261 Go to UniProtKB: P40261 | |||||
PHAROS: P40261 GTEx: ENSG00000166741 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P40261 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| 9XL Query on 9XL | E [auth C], K [auth A], N [auth B], P [auth D] | (2S)-2-amino-4-([3-(3-carbamoylphenyl)prop-2-yn-1-yl]{[(1R,2R,3S,4R)-4-(4-chloro-7H-pyrrolo[2,3-d]pyrimidin-7-yl)-2,3-dihydroxycyclopentyl]methyl}amino)butanoic acid C26 H29 Cl N6 O5 JIYDUPLVTSNGNC-BLCKVISQSA-N |  | ||
| PEG Query on PEG | F [auth C] G [auth C] H [auth C] I [auth C] J [auth C] | DI(HYDROXYETHYL)ETHER C4 H10 O3 MTHSVFCYNBDYFN-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 60.821 | α = 110.07 |
| b = 62.596 | β = 103.82 |
| c = 79.781 | γ = 103.85 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| SCALEPACK | data scaling |
| PHASER | phasing |
| PDB_EXTRACT | data extraction |
| HKL-2000 | data reduction |
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Institutes of Health/National Institute of General Medical Sciences (NIH/NIGMS) | United States | 1R01GM127896-01 |
| National Institutes of Health/National Institute of General Medical Sciences (NIH/NIGMS) | United States | 1R01GM127884-01A1 |
| National Institutes of Health/National Institute Of Allergy and Infectious Diseases (NIH/NIAID) | United States | 1R01AI127793 |
| National Institutes of Health/National Institute Of Allergy and Infectious Diseases (NIH/NIAID) | United States | R01GM117275 |
| National Institutes of Health/National Cancer Institute (NIH/NCI) | United States | U01CA214649-01 |