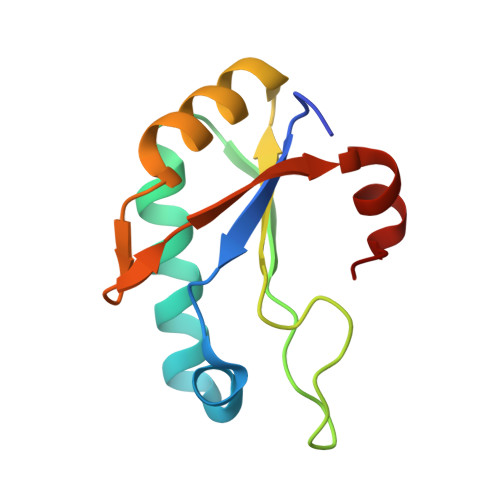
A UHM-ULM interface with unusual structural features contributes to U2AF2 and SF3B1 association for pre-mRNA splicing.
Galardi, J.W., Bela, V.N., Jeffery, N., He, X., Glasser, E., Loerch, S., Jenkins, J.L., Pulvino, M.J., Boutz, P.L., Kielkopf, C.L.(2022) J Biological Chem 298: 102224-102224
- PubMed: 35780835
- DOI: https://doi.org/10.1016/j.jbc.2022.102224
- Primary Citation of Related Structures:
7SN6 - PubMed Abstract:
During spliceosome assembly, the 3' splice site is recognized by sequential U2AF2 complexes, first with Splicing Factor 1 (SF1) and second by the SF3B1 subunit of the U2 small nuclear ribonuclear protein particle. The U2AF2-SF1 interface is well characterized, comprising a U2AF homology motif (UHM) of U2AF2 bound to a U2AF ligand motif (ULM) of SF1. However, the structure of the U2AF2-SF3B1 interface and its importance for pre-mRNA splicing are unknown. To address this knowledge gap, we determined the crystal structure of the U2AF2 UHM bound to a SF3B1 ULM site at 1.8-Å resolution. We discovered a distinctive trajectory of the SF3B1 ULM across the U2AF2 UHM surface, which differs from prior UHM/ULM structures and is expected to modulate the orientations of the full-length proteins. We established that the binding affinity of the U2AF2 UHM for the cocrystallized SF3B1 ULM rivals that of a nearly full-length U2AF2 protein for an N-terminal SF3B1 region. An additional SF3B6 subunit had no detectable effect on the U2AF2-SF3B1 binding affinities. We further showed that key residues at the U2AF2 UHM-SF3B1 ULM interface contribute to coimmunoprecipitation of the splicing factors. Moreover, disrupting the U2AF2-SF3B1 interface changed splicing of representative human transcripts. From analysis of genome-wide data, we found that many of the splice sites coregulated by U2AF2 and SF3B1 differ from those coregulated by U2AF2 and SF1. Taken together, these findings support distinct structural and functional roles for the U2AF2-SF1 and U2AF2-SF3B1 complexes during the pre-mRNA splicing process.
- Center for RNA Biology, Department of Biochemistry and Biophysics, University of Rochester School of Medicine and Dentistry, Rochester, New York, USA.
Organizational Affiliation: