Design, Synthesis, and Characterization of TNP-2198, a Dual-Targeted Rifamycin-Nitroimidazole Conjugate with Potent Activity against Microaerophilic and Anaerobic Bacterial Pathogens.
Ma, Z., He, S., Yuan, Y., Zhuang, Z., Liu, Y., Wang, H., Chen, J., Xu, X., Ding, C., Molodtsov, V., Lin, W., Robertson, G.T., Weiss, W.J., Pulse, M., Nguyen, P., Duncan, L., Doyle, T., Ebright, R.H., Lynch, A.S.(2022) J Med Chem 65: 4481-4495
- PubMed: 35175750
- DOI: https://doi.org/10.1021/acs.jmedchem.1c02045
- Primary Citation of Related Structures:
7RWI - PubMed Abstract:
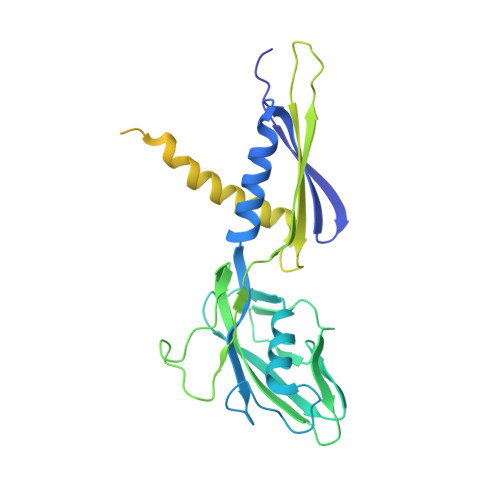
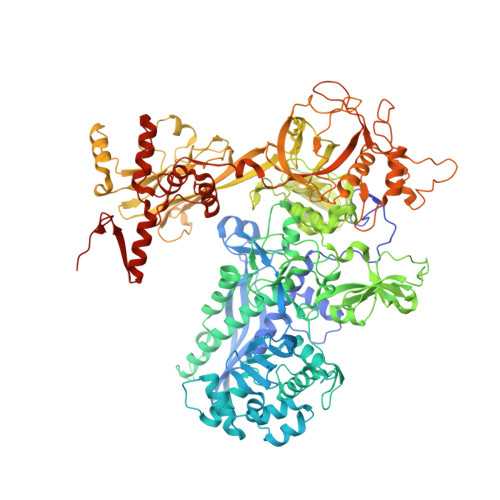
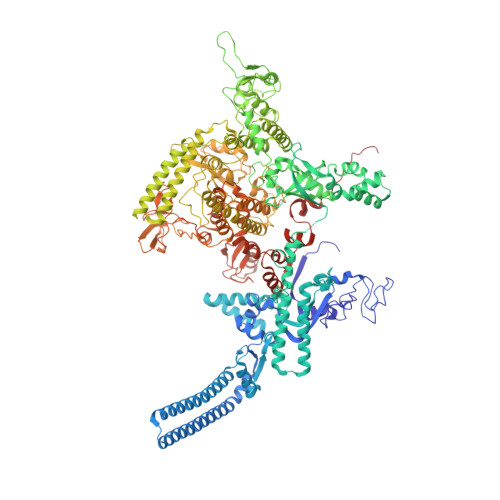
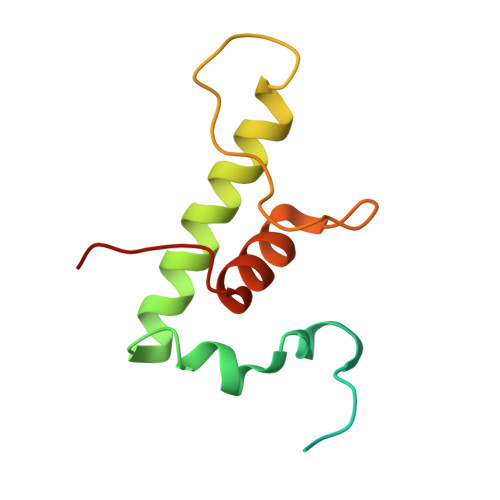
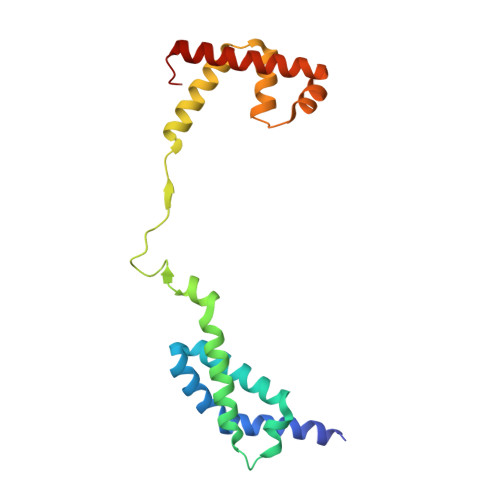
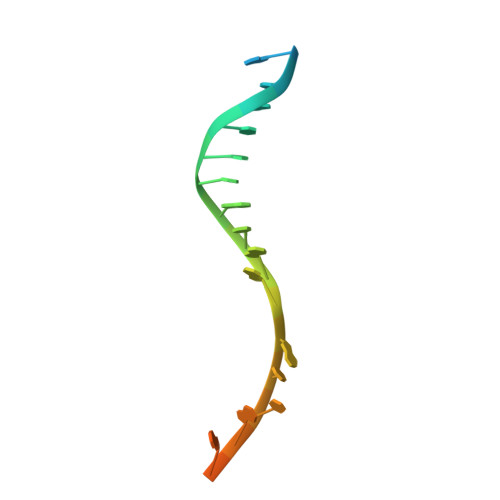
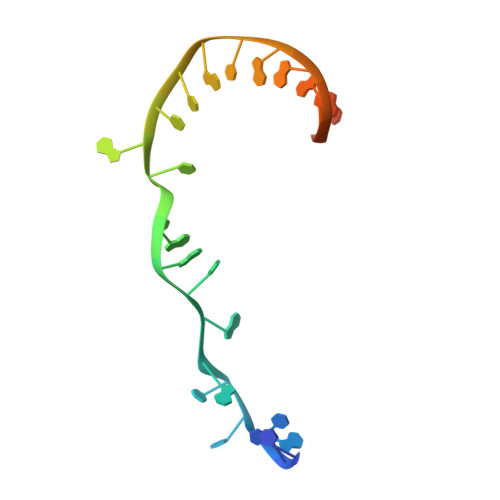
TNP-2198, a stable conjugate of a rifamycin pharmacophore and a nitroimidazole pharmacophore, has been designed, synthesized, and evaluated as a novel dual-targeted antibacterial agent for the treatment of microaerophilic and anaerobic bacterial infections. TNP-2198 exhibits greater activity than a 1:1 molar mixture of the parent drugs and exhibits activity against strains resistant to both rifamycins and nitroimidazoles. A crystal structure of TNP-2198 bound to a Mycobacterium tuberculosis RNA polymerase transcription initiation complex reveals that the rifamycin portion of TNP-2198 binds to the rifamycin binding site on RNAP and the nitroimidazole portion of TNP-2198 interacts directly with the DNA template-strand in the RNAP active-center cleft, forming a hydrogen bond with a base of the DNA template strand. TNP-2198 is currently in Phase 2 clinical development for the treatment of Helicobacter pylori infection, Clostridioides difficile infection, and bacterial vaginosis.
- TenNor Therapeutics Ltd, 218 Xinghu Street, Suzhou Industrial Park, Suzhou 215123, China.
Organizational Affiliation: