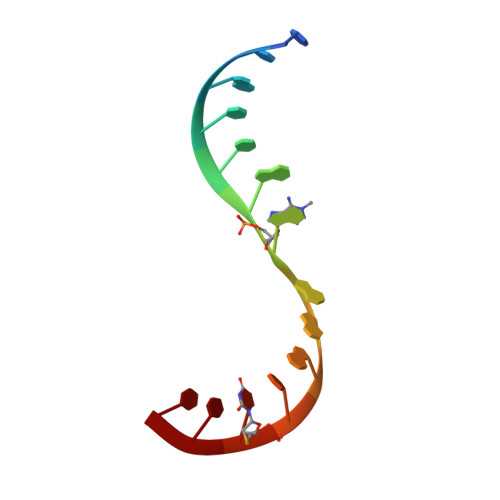
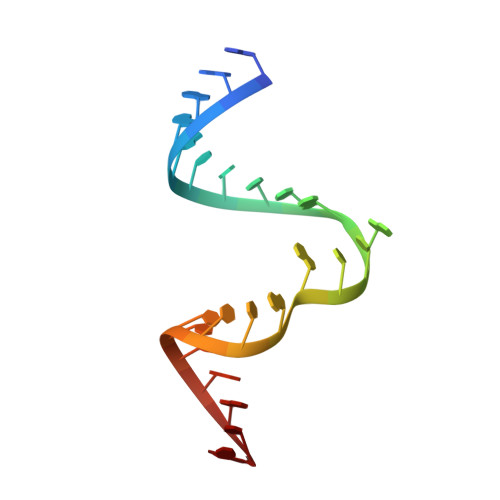
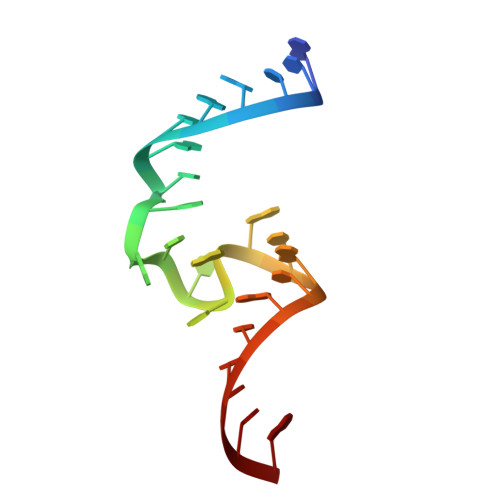
Structure and mechanism of the methyltransferase ribozyme MTR1.
Scheitl, C.P.M., Mieczkowski, M., Schindelin, H., Hobartner, C.(2022) Nat Chem Biol 18: 547-555
- PubMed: 35301481
- DOI: https://doi.org/10.1038/s41589-022-00976-x
- Primary Citation of Related Structures:
7Q7X, 7Q7Y, 7Q7Z, 7Q80, 7Q81, 7Q82 - PubMed Abstract:
RNA-catalyzed RNA methylation was recently shown to be part of the catalytic repertoire of ribozymes. The methyltransferase ribozyme MTR1 catalyzes the site-specific synthesis of 1-methyladenosine (m 1 A) in RNA, using O 6 -methylguanine (m 6 G) as a methyl group donor. Here, we report the crystal structure of MTR1 at a resolution of 2.8 Å, which reveals a guanine-binding site reminiscent of natural guanine riboswitches. The structure represents the postcatalytic state of a split ribozyme in complex with the m 1 A-containing RNA product and the demethylated cofactor guanine. The structural data suggest the mechanistic involvement of a protonated cytidine in the methyl transfer reaction. A synergistic effect of two 2'-O-methylated ribose residues in the active site results in accelerated methyl group transfer. Supported by these results, it seems plausible that modified nucleotides may have enhanced early RNA catalysis and that metabolite-binding riboswitches may resemble inactivated ribozymes that have lost their catalytic activity during evolution.
- Institute of Organic Chemistry, Julius-Maximilians-Universität Würzburg, Am Hubland, Würzburg, Germany.
Organizational Affiliation: